Writers: Francisco Trivinho-Strixino; Donizete X. da Silva; Carlos O. Paiva-Santos; Ernesto C. Pereira.
Keywords: Valvemetals; ZrO2; Anodicfilms; Anodic breakdown; Microstructure; Phasetransformation; Plasma electrolyticoxidation(PEO)
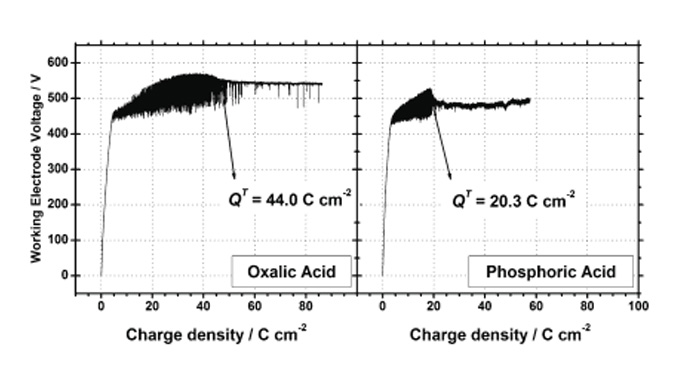
Abstract: Plasma electrolytic oxidation (PEO) is a coating procedure that utilises anodic oxidation in aqueous electrolytes above the dielectric breakdown voltage to produce oxide coatings that have specific properties. These conditions facilitate oxide formation under localised high temperatures and pressures that originate from short-lived microdischarges at sites over the metal surface and have fast oxide volume expansion. Anodic ZrO2 films were prepared by subjecting metallic zirconium to PEO in acid solutions (H2C2O4 and H3PO4) using a galvanostatic DC regime. The ZrO2 microstructure was investigated in films that were prepared at different charge densities. During the anodic breakdown, an important change in the amplitude of the voltage oscillations at a specific charge density was observed (i.e., the transition charge density (QT)). We verified that this transition charge is a monotonic function of both the current density and temperature applied during the anodisation, which indicated that QT is an intrinsic response of this system. The oxide morphology and microstructure were characterised using SEM and X-ray diffraction experiments (XRD) techniques. X-ray diffraction analysis revealed that the change in voltage oscillation was correlated with oxide microstructure changes during the breakdown process.