Writers: A.L. Horovistiz, R.A. Rocha, E.N.S. Muccillo
Keywords:Powders; chemical preparation; Ionic conductivity; CeO2; Fuel cells
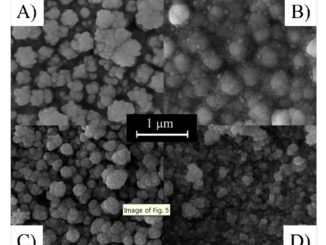
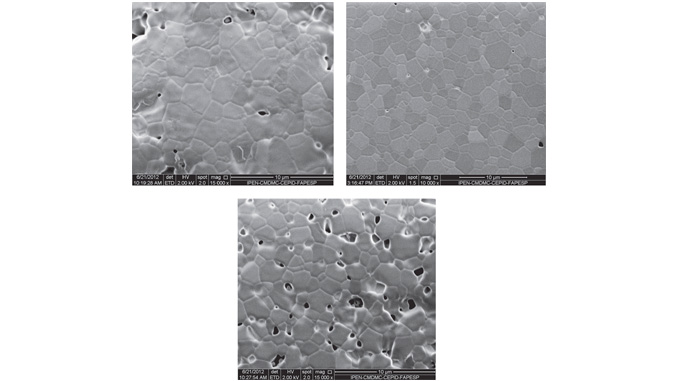
Abstract: The effects of small amounts of bismuth and sodium on the electrical conductivity of gadolinia-doped ceria are investigated. Bulk specimens of nominally pure Ce0.9Gd0.2O1.9 and Ce0.8Gd0.19M0.01O1.9 with M¼Bi or Na were prepared by the cation complexation technique. Raman spectra of sintered specimens show a prominent band at approximately 464 cm1 assigned to the cubic fluorite-type lattice of cerium oxide. Both additives promoted changes in the microstructure of samples sintered at 1723 K. Bi-containing samples exhibit low porosity along with angular grains with mean grain size of about 1.7 mm compared to 1.4 mm of pure gadolinia-doped ceria. Addition of Na to gadolinia-doped ceria reduced the mean grain size (1.0 mm) but porosity increased drastically. Electrical conductivity measurements were used to study mass transport. Analysis of impedance spectroscopy data shows that the apparent activation energy for conduction is slightly lower in samples containing the additives explaining the higher grain and grain boundary conductivities of the ternary systems.