Writers: A.G. Sato, D.P. Volanti, D.M. Meira, S. Damyanova, E. Longo, J.M.C. Bueno
Keywords: Ethanol dehydrogenation; Cu/ZrO2 catalysts; Ethyl acetate; Characterization
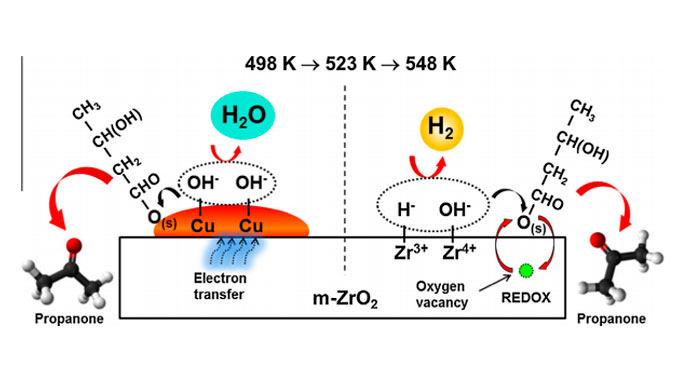
Abstract: The effect of amorphous (am-), monoclinic (m-), and tetragonal (t-) ZrO2 phase on the physicochemical and catalytic properties of supported Cu catalysts for ethanol conversion was studied. The electronic parameters of Cu/ZrO2 were determined by in situ XAS, and the surface properties of Cu/ZrO2 were defined by XPS and DRIFTS of CO-adsorbed. The results demonstrated that the kind of ZrO2 phase plays a key role in the determination of structure and catalytic properties of Cu/ZrO2 catalysts predetermined by the interface at Cu/ZrO2. The electron transfer between support and Cu surface, caused by the oxygen vacancies at m-ZrO2 and am-ZrO2, is responsible for the active sites for acetaldehyde and ethyl acetate formation. The highest selectivity to ethyl acetate for Cu/m-ZrO2 catalyst up to 513 K was caused by the optimal ratio of Cu0 /Cu+ species and the high density of basic sites (O2) associated with the oxygen mobility from the bulk m-ZrO2.
DOI: 10.1016/j.jcat.2013.06.022




