Writers: Alana A. Zulke; Roberto Matos; Ernesto C. Pereira
Keywords: Metallic multilayer; Electrocatalysis; Titanium modified electrode; Methanol oxidation reaction
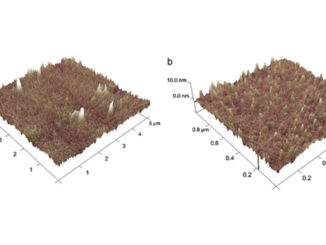
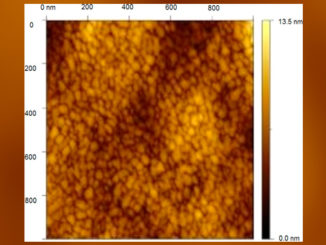
Abstract: Multilayered Pt/Ir/Pt films were electrodeposited over titanium substrates, controlling the film thickness by the deposition charge. Two probe molecules were used to perform the evaluation of catalytic activity, CH3OH and CO. Using a 22 factorial design with a central point, optimized intrinsic catalytic activity was obtained over the Ti/Pt25mC/Ir6mC/Pt electrode. An enhancement of the current density of up to 2.76 times was observed for the methanol oxidation reaction (MOR) compared with the reference sample (Ti/Pt electrodes), even though both samples had the same electroactive area. In addition to the methanol oxidation voltammetry, CO stripping voltammetry suggests that MM-like systems are less susceptible to the catalyst-poisoning phenomenon compared to the Ti/Pt ones. Impedance spectroscopy was mainly used to monitor the charge transference resistance (Rct), whose results showed a clear Rct reduction for the MOR process over MMs compared with Ti/Pt electrodes, corroborating the previous observations regarding the general catalytic improvement.
DOI: 10.1016/j.electacta.2013.05.027