Writers: Raquel Kely Bortoleto-Bugs, Talita Mazon, Márcio Tarozzo Biasoli, Aristides Pavani Filho, Jacobus Willibrordus Swart, and Milton Roque Bug
Keywords: Nanoparticles
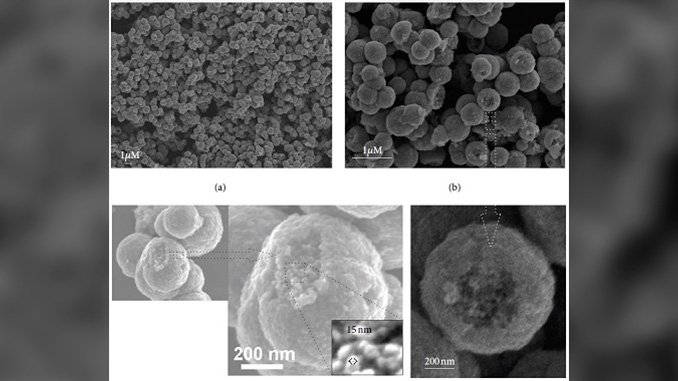
Abstract: Self-assembly procedure is employed to synthesize colloidal copper nanoparticles (ccNPs) with cationic surfactant in an environmentally friendly method. Scanning electron microscopy images provide a clear view of the ccNPs formed having an approximate size of 15 nm. The X-ray diffraction reveals that the ccNPs have the two types of copper oxide as well as the metallic copper. The new procedure shows that the cationic surfactant CTAB plays an important role in the understanding and development of self-assembly. There is a strong relationship between the ccNPs formation with the critical micelle concentration of the CTAB which influences both shape and size. The outcomes allowed the development of a molecular model for the ccNPs synthesis showing that the CTAB monomer on the surface has the function of a molecular velcro making the linkage of ccNPs to form an agglomerate with size around 600 nm. Finally, with the emerging new technologies, the synthesis of copper oxide takes a new perspective for their applicability in diverse integrated areas such as the flexible electronics and energy.