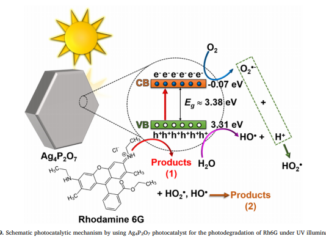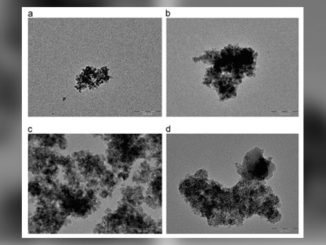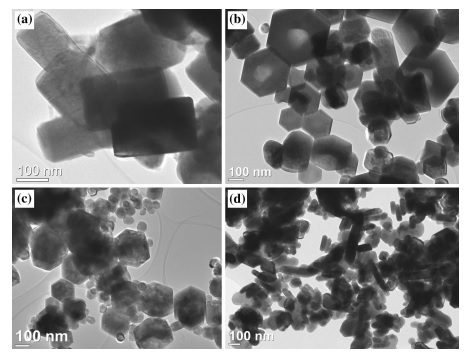
Writers: D. Lukovic Golic, J. C irkovic, M. Scepanovic, T. Sreckovic, E. Longo, J. A. Varela, N. Daneu, V. Stamenkovic, G. Brankovic, Z. Brankovic
Keywords: ZnO Nano- and submicron particles; Solvothermal synthesis; Growth mechanism; Structure ordering; Photoluminescence Int
Abstract: Nano- (30–60 nm) and submicron (100–350 nm) ZnO particles were synthesized using solvothermal method at 200 C from an ethanolic solution of zinc acetate dihydrate, applying different reaction conditions, i.e., pH value of precursor and time of the reaction. The X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), UV–vis diffuse reflectance (DR), Raman spectroscopy, and photoluminescence (PL) spectroscopy have been employed for characterization of synthesized ZnO powders. It was shown that the structural, morphological, and optical properties are largely determined by reaction conditions during solvothermal synthesis. The particle crystallinity improves with the decrease of pH value and/or the increase of time of the reaction. The Raman and PL spectra analyses indicate that the oxygen interstitials are dominant intrinsic defects in solvothermally synthesized ZnO powders. It was observed that concentration of defects in wurtzite ZnO crystal lattices slightly changes with the variation of pH value of the precursor and time of the solvothermal reaction. The correlation between structural ordering and defect structure of particles and corresponding growth processes was discussed.