Writers: M.D.P. Silva and R.F. Gonçalves and I.C. Nogueira and V.M. Longo and L. Mondoni and M.G. Moron and Y.V. Santana and E. Longo
Keywords: Ag2WO4; Ag2MoO4; Photoluminescence; Morphology; Raman spectroscopy
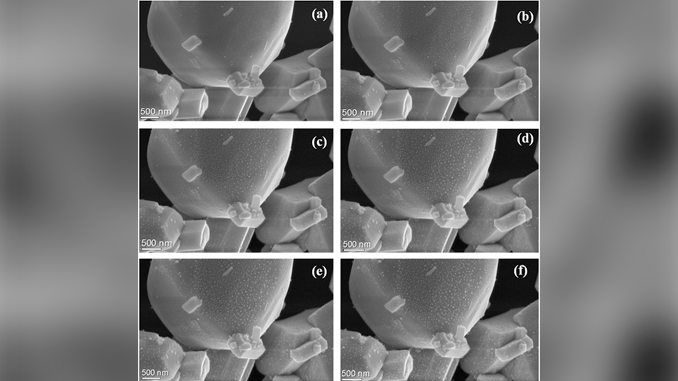
Abstract: Ag2W1 -xMoxO4 (x = 0.0 and 0.50) powders were synthesized by the co-precipitation (drop-by-drop) method and processed using a microwave-assisted hydrothermal method. We report the real-time in situ formation and growth of Ag filaments on the Ag2W1 -xMoxO4 crystals using an accelerated electron beam under high vacuum. Various techniques were used to evaluate the influence of the network-former substitution on the structural and optical properties, including photoluminescence (PL) emission, of these materials. X-ray diffraction results confirmed the phases obtained by the synthesis methods. Raman spectroscopy revealed significant changes in local order–disorder as a function of the network-former substitution. Field-emission scanning electron microscopy was used to determine the shape as well as dimensions of the Ag2W1 -xMoxO4heterostructures. The PL spectra showed that the PL-emission intensities of Ag2W1 -xMoxO4 were greater than those of pure Ag2WO4, probably because of the increase of intermediary energy levels within the band gap of the Ag2W1 -xMoxO4 heterostructures, as evidenced by the decrease in the band-gap values measured by ultraviolet–visible spectroscopy.