Writers: Alencar, LDS; Mesquita, A; Feitosa, CAC; Balzer, R; Probst, LFD; Batalha, DC; Rosmaninho, MG; Fajardo, HV; Bernardi, MIB
Keywords: BaMoO4; BaWO4; Microwave-assisted hydrothermal; Polymeric precursor; volatile organic-compounds; oxygen mobility; photoluminescence properties; optical characterization; metal tungstates; carbon-monoxide; low-temperature; mixed oxides; gold; removal
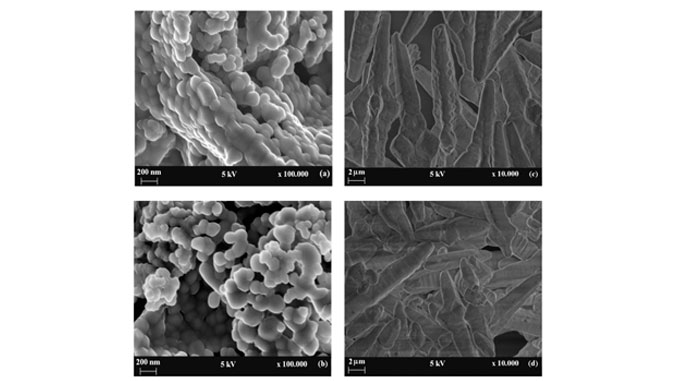
Abstract: Barium molybdate and Barium tungstate are important materials due their photoluminescent properties and they also have catalysis and photocatalysis applications. In this work, powders of these compounds were prepared by microwave-assisted hydrothermal (MAH) method and polymeric precursor method (PPM) and their structural and optical properties were studied. Furthermore, these materials were employed as solid catalysts towards gas phase toluene oxidation reactions. X-ray diffraction confirms the purity of materials at both preparation methods and reveals a preferential growth when the powders are prepared by MAH due polymeric agents and processing using microwave, which was confirmed by Field emission scanning electron microscopy. Photoluminesce emission was attributed to the charge-transfer transitions within the [WO4](2-) and [MoO4](2-) complexes. The H-2 Temperature-Programmed Reduction (H-2-TPR), Oz-chemisorption and extended X-ray absorption fine structure (EXAFS) results indicated that BaWO4 samples, compared with BaMoO4 samples, have higher oxygen mobility and oxygen vacancies that appear as key factors for the achievement of better catalytic performances.
DOI: 10.1016/j.ceramint.2016.12.096