Writers: Martin S. Barbosa and Pedro H. Suman and Jae J. Kim and Harry L. Tuller and José A. Varela and Marcelo O. Orlandi
Keywords: Gas sensors; SnO; Catalyst; Impedance spectroscopy; Sensitization
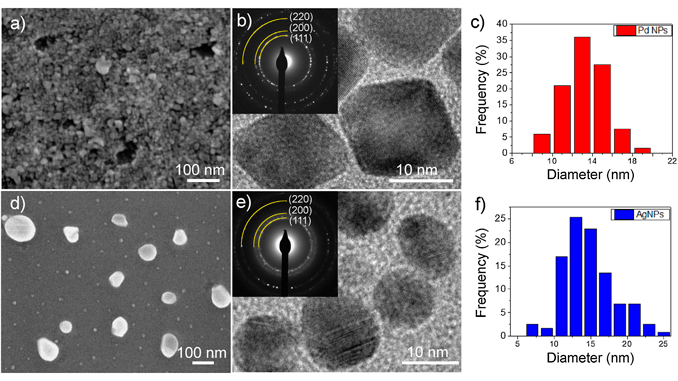
Abstract: The gas sensor response of tin monoxide micro-disks, functionalized with noble metal nanoparticles (Pd and Ag), to NO2, H-2 and CO were studied by monitoring changes in their resistance upon exposure to the various gases. The tin monoxide, with unusually low Sn oxidation state, was synthetized by carbothermal reduction. Surface modification by Pd and Ag catalysts was achieved by coating the micro-disks by metallic nanoparticle dispersions, prepared by the polyol reduction process, followed by thermal treatment. SEM and TEM analysis showed nanoparticles to be well-dispersed over the SnO surfaces. The decorated SnO micro-disks exhibited high sensor response to reducing gases such as H-2 and CO. On the other hand, the catalytic particles tended to reduce the sensor response to oxidizing gases such as NO2. The catalytic activity of Pd nanoparticles was tied to chemical sensitization while that of Ag nanoparticles to electronic sensitization. Impedance spectroscopy enabled deconvolution of different contributions to the sensor response with only the Ag-decorated specimens exhibiting two RC time constants. Thus, in contrast to undecorated and Pd-decorated specimens, nearly 80% of Ag modified SnO’s response to H-2 was controlled by changes in the interface between particles and disks. Sensor response to H-2 was optimal at higher temperatures (300 degrees C), NO2 at 200 degrees C while that for Pd-decorated materials; maximum sensor response to CO was observed at lower temperatures (under 150 degrees C), where CO absorption by metal nanoparticles is favored.