Accessible ring opening metathesis and atom transfer radical polymerization catalysts based on dimethyl sulfoxide ruthenium(II) complexes bearing N-heterocyclic carbene ligands
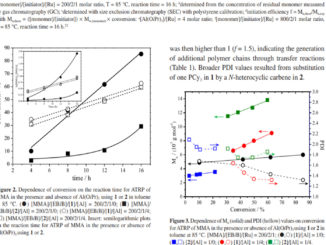
Abstract: Dimethyl sulfoxide ruthenium(II) complexes of N-heterocyclic carbenes derived from cycloalkylamines (cycloalkyl = cyclopentyl (1a), cyclohexyl (1b), cycloheptyl (1c), and cyclooctyl (1d)) were synthesized: [RuCl2(S-dmso)2(IPent)] (2a), [RuCl2(S-dmso)2(IHex)] (2b), [RuCl2(S-dmso)2(IHept)] (2c), and [RuCl2(S-dmso)2(IOct)] (2d). The imidazolium salts 1a–1d were characterized by FTIR, UV–vis, and 1H and 13C NMR spectroscopy, while their respective dimethyl sulfoxide ruthenium(II) complexes (2a–2d) were characterized by elemental analysis, FTIR, UV–vis, 1H and 13C NMR, and cyclic voltammetry. The complexes 2a–2d were evaluated as catalytic precursors for ROMP of norbornene (NBE) and for ATRP of methyl methacrylate (MMA). The polynorbornene (polyNBE) syntheses via ROMP using the complexes 2a–2d as pre-catalysts were evaluated under reaction conditions of [EDA]/[Ru] = 28 (5 μL), [NBE]/[Ru] = 5000 at 50 °C as a function of time. The polymerization of MMA via ATRP was conducted using the complexes 2a–2d in the presence of ethyl 2-bromoisobutyrate (EBiB) as the initiator. All tests were using the molar ratio [MMA]/[EBiB]/[Ru] = 1000/2/1 and conducted at 85 °C. The linear correlation of ln([MMA]0/[MMA]) and time clearly indicates that the concentration of radicals remains constant during the polymerization and that the ATRP of MMA mediated by 2a–2d proceeds in a controlled manner.
Authors: André H.S.Idehara, Patrik D. S. Gois, Henrique Fernandez, Beatriz E. Goi, Antonio E. H. Machado, Benedito S. Lima-Neto, Valdemiro P. Carvalho Jr.
Molecular Catalysis
Volume 448, April 2018, Pages 135-143
DOI: https://doi.org/10.1016/j.mcat.2018.01.032