Nucleophilic Addition of Amines to Ruthenium Carbenes: ortho-(Alkynyloxy)benzylamine Cyclizations towards 1,3-Benzoxazines
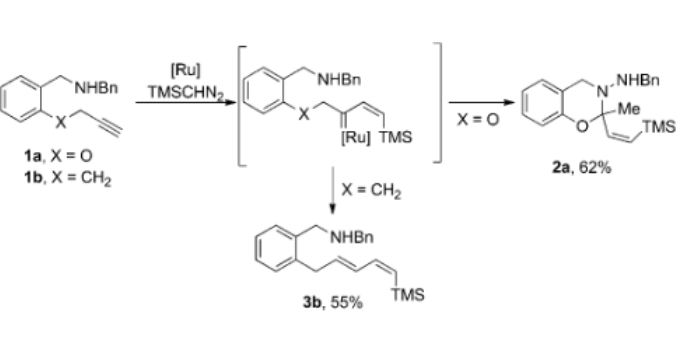
Abstract: A new ruthenium-catalyzed cyclization of ortho-(alkynyloxy)benzylamines to dihydro-1,3-benzoxazines is reported. The cyclization is thought to take place via the vinyl ruthenium carbene intermediates which are easily formed from [Cp*RuCl(cod)] and N2CHSiMe3. The mild reaction conditions and the efficiency of the procedure allow the easy preparation of a broad range of new 2-vinyl-2-substituted 1,3-benzoxazine derivatives. Rearrangement of an internal C(sp) in the starting material into a tetrasubstituted C(sp(3)) atom in the final 1,3-benzoxazine is highly remarkable.
Author(s): Gonzalez-Rodriguez, C; Suarez, JR; Varela, JA; Saa, C
ANGEWANDTE CHEMIE-INTERNATIONAL EDITION
Volume: 54 Pages: 2724-2728 Published: FEB 23 2015
DOI: 10.1002/anie.201410284