The effect of solution pH on the oscillatory electro-oxidation of methanol
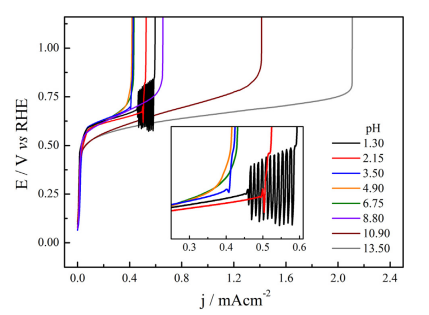
Abstract: The methanol electro-oxidation reaction (MER) was studied in a wide pH range under voltammetric and oscillatory regimes. Employing buffered hydrogen phosphates solutions, it was demonstrated that increasing the pH MER is firstly inhibited in the region up to 4-5 but after this point starts to increase reaching currents 5 times bigger than those in acidic media. Potential oscillations during MER are restricted to the pH < 3.5. While the oscillations frequency and shape change slightly in the studied pH range, the amplitude and poisoning rate, calculated in terms of dE/dt, are severely affected by the pH, culminating in the extinguishment of oscillations.
Author(s): Melle, GB; Hartl, FW; Varela, H; Sitta, E
JOURNAL OF ELECTROANALYTICAL CHEMISTRY
Volume: 826 Pages: 164-169 Published: OCT 1 2018
PDF: The effect of solution pH on the oscillatory electro-oxidation of methanol
DOI: 10.1016/j.jelechem.2018.08.033