Strongly Red-Shifted Photoluminescence Band Induced by Molecular Twisting in Cyanine (Cy3) Dye Films
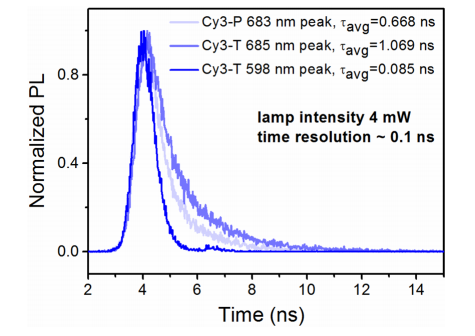
Abstract: Cyanine dye molecules, used as monomers or in aggregate form, find interesting applications in optoelectronic devices. Among the various aggregate species incorporating organic dyes, centrosymmetric dimers are known as nonluminescent. They can act as exciton quenchers due to a low-energy optically forbidden excited state. In this study, however, we, show that a dimer species in thin films exhibits efficient and strongly red-shifted photoluminescence. When the films were excited, a monomer emission at 590 nm along with a second emission peak at 680 nm was observed. A close relation between the dye concentration and the emission showed that a new emission at 680 nm corresponds to the dimer emission. Circular dichroism (CD) spectroscopy reveals that a fraction of the dimers exist in a twisted dimer configuration. Stable, long-lived, and quenchable fluorescence with high quantum yield is attributed to this (Timer emission.
Author(s): Anantharaman, SB; Yakunin, S; Peng, CY; Vismara, MVG; Graeff, CFO; Nuesch, FA; Jenatsch, S; Hany, R; Kovalenko, MV; Heier, J
JOURNAL OF PHYSICAL CHEMISTRY C
Volume: 121 Pages: 9587-9593 Published: MAY 4 2017
PDF: Strongly Red-Shifted Photoluminescence Band Induced by Molecular Twisting in Cyanine (Cy3) Dye Films
DOI: 10.1021/acs.jpcc.7b01412