Nanostructured ZnS:Cu phosphor: Correlation between photoluminescence properties and local structure
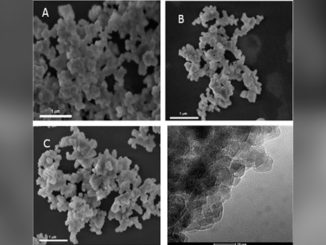
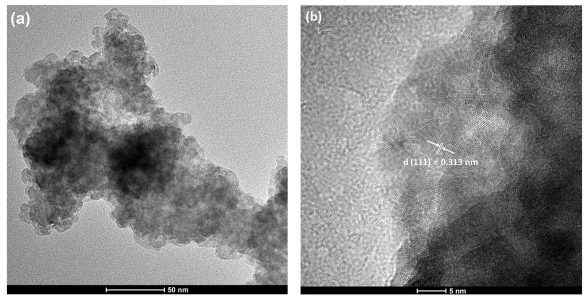
Abstract: Zinc sulfide (ZnS) is a II-VI inorganic semiconductor material and has received remarkable attention because of fundamental physical properties, versatility, nontoxicity, chemical stability and has been widely applied to make numerous optical devices as phosphor material. As a wide band gap semiconductor, ZnS can easily host different metal ions as luminescent center to improve or modify its structural and optical performances such as Cu atoms. In this paper, ZnS:Cu nanoparticles were synthesized by solvothermal method and the photoluminescence and the structural at long- and local-range properties were characterized. According to X-ray diffraction results, ZnS sample crystallizes with the cubic zinc blende structure (F-43m space group) without spurious phases. Images from transmission electron microscope depict the morphology of ZnS particles as round shape and average size value lower than 5 nm. Theoretical and experimental X-ray absorption near edge structure (XANES) spectra at Zn K-edge suggest the incorporation of Cu atoms into the ZnS host and indicate the occurrence of Zn and S vacancies, which are confirmed by extended X-ray absorption fine structure (EXAFS) fit results. These native defects are related to a red-shift observed in the peak emission of photoluminescence (PL) spectrum for ZnS sample, which is centered at similar to 504 nm. An emission in orange-red region is observed as Cu is incorporated in the ZnS host matrix that is attributed to the transition from the T-4(1) level to the (6)A(1) level. Moreover, the intensity of the emissions due to zinc vacancies and interstitial zinc in the deconvoluted PL spectrum is in accordance with results determined in X-ray absorption spectroscopy characterization.
Author (s): Curcio, AL; da Silva, LF; Bernardi, MIB; Longo, E; Mesquita, A
JOURNAL OF LUMINESCENCE
Volume: 206 Pages: 292-297 Published: FEB 2019
PDF: Nanostructured ZnS Cu phosphor Correlation between photoluminescence properties and local structure
DOI: 10.1016/j.jlumin.2018.10.073