Hydrothermal synthesis, structural characterization and photocatalytic properties of beta-Ag2MoO4 microcrystals: Correlation between experimental and theoretical data
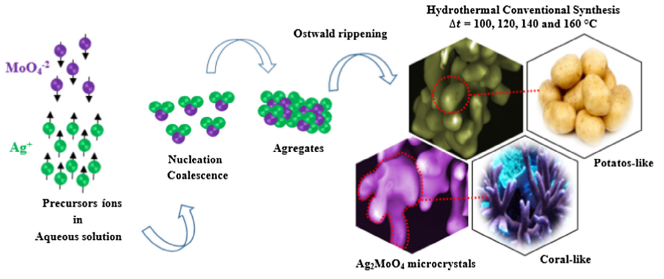
Abstract: In this paper, we report about hydrothermal synthesis, structural characterization and photocatalytic properties of beta-silver molybdate (beta-Ag2MoO4) microcrystals obtained at different temperatures (100, 120, 140 and 160 degrees C) for 2 h. These crystals were characterized structurally using X-ray diffraction (XRD), X-ray fluorescence, Rietveld refinement, micro-Raman (m-Raman) and Fourier-transform infrared (FT-IR) spectroscopies. Experimental and theoretical band gap values were correlated by ultraviolet-visible (UV-Vis) diffuse reflectance spectroscopy and periodic first-principles calculations in the framework of density functional theory (DFT) with the B3LYP-D3 hybrid functional. The crystals morphology was observed through field-emission scanning electron microscopy (FE-SEM) images. The photocatalytic properties of these crystals were investigated for degradation of rhodamine B (RhB) dye under UV-light. XRD patterns and Rietveld refinement data indicate that all crystals exhibit a spinel-type cubic structure with space group (Fd3’m) formed by tetrahedral [MoO4] clusters and distorted octahedral [AgO6] clusters. m-Raman spectra exhibited five Raman-active modes in a range from 50 to 1000 cm(-1), while FT-IR spectra have three infrared active modes in a range from 400 to 1100 cm(-1). The experimental results from Raman and IR modes are in reasonable agreement with theoretically calculated results. Experimental UV-Vis spectra indicate a decrease in optical band gap (E-ga(p )= 3.35 eV to 3.32 eV) with the temperature rise. The calculated band structure revealed an indirect optical band gap (E-gap) of approximately 3.94 eV. Moreover, theoretical calculations based on density of states and electron density maps were employed to understand the polarization phenomenon induced by structural defects in the beta-Ag2MoO4 microcrystals. FE-SEM images revealed that the increase of processing temperatures promotes a change in shape of microcrystals from potatoes-like to coral-like. Finally, photocatalytic measures to degradation of the RhB dye resulted in the best catalytic performance for beta-Ag2MoO4 microcrystals synthesized at temperatures of 120 and 140 degrees C, corresponding to 97.3% and 96.8% in the photodegradation of RhB dye under UV-light up to 2 h. The stability of the beta-Ag2MoO4 was investigated by reusing, resulting in f 97.2, 93.9 and 78.8% degradation of the RhB dye for the first, second and third cycle, respectively. (C) 2018 Production and hosting by Elsevier B.V. on behalf of King Saud University.
Author(s): Sousa, GD ; Nobre, FX ; Araujo, EA ; Sambrano, JR ; Albuquerque, AD ; Binda, RD ; Couceiro, PRD ; Brito, WR ; Cavalcante, LS ; Santos, MRDC ; de Matos, JME
ARABIAN JOURNAL OF CHEMISTRY
Volume: 13 Edição: 1 Páginas: 2806-2825 Published: JAN 2020
DOI: 10.1016/j.arabjc.2018.07.011