Investigation of the Fe-Mo electrodeposition from sorbitol alkaline bath and characterization of the films produced
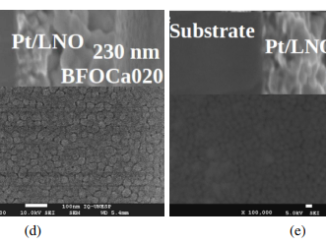
Abstract: The electrodeposition of Fe-Mo alloys from an alkaline solution containing sorbitol as the complexing agent for Fe(III) ions was studied. XRD analysis of the film produced by reduction of [MoO4]2- anions at a copper substrate showed the formation of MoO2. The presence of the intermediate Fe(II) species was detected by electrolysis from an Fe-Mo solution and Visible spectroscopy of the final solution in the presence of 1,10-phenanthroline. The Visible spectrum showed a broad absorption (λmax = 514 nm) typical of the [Fe(phen)3]2+ complex. Under our conditions, the Fe-Mo alloy was formed from reduction of [Fe(OH)2(C6H12O6)2]3- to [Fe(OH)2(C6H12O6)2]4-, reduction of [MoO4]2- to molybdenum (IV) oxide/hydroxide and its further reduction to Mo0concomitant with [Fe(OH)2(C6H12O6)2]4−ads to Fe0. Photomicrographs of the Fe-Mo electrodeposits produced with qd 5.30Ccm−2 showed that they were smooth or contained cracks, depending on the deposition potential (Ed). Chemical composition analysis by EDXS of Fe-Mo electrodeposits (qd 5.30Ccm−2) showed that deposits containing 5.5 at% (18.8 wt%) Mo could be produced at Ed −1.40 V and similar values were obtained at more negative potentials. X-Ray diffraction indicated that the Fe-Mo coatings were composed of Fe3Mo and Fe2MoO4 phases, regardless of Ed. The presence of the Fe2MoO4 phase on the sample surface could explain the observation of Fe2+ and Mo4+ states by XPS, although the occurrence of others crystalline oxidephases, such as FeMoO4 and Fe2(MoO4)3 could not be discarded. The crystal size value varied from ∼47 nm to ∼37 nm, when Ed changed to more negative values.
Author(s): Zacarin, Maria G.; de Brito, Matheus M.; Barbano, Elton P.; et al.
Journal of Alloys and Compounds
DOI: https://doi.org/10.1016/j.jallcom.2018.04.020