Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression
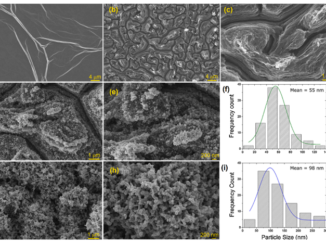
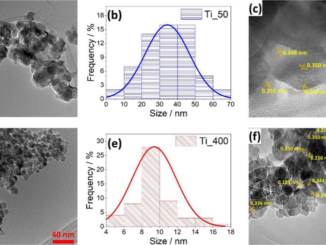
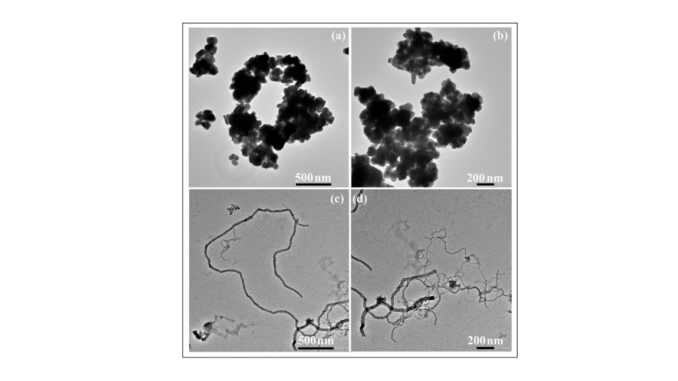
Abstract: Carbon nanofibers (CNFs) are one-dimensional nanomaterials with excellent physical and broad-spectrum antimicrobial properties characterized by a low risk of antimicrobial resistance. Silver nanoparticles (AgNPs) are antimicrobial metallic nanomaterials already used in a broad range of industrial applications. In the present study these two nanomaterials were characterized by Raman spectroscopy, transmission electron microscopy, zeta potential, and dynamic light scattering, and their biological properties were compared in terms of cytotoxicity, proliferation, and gene expression in human keratinocyte HaCaT cells. The results showed that both AgNPs and CNFs present similar time-dependent cytotoxicity (EC50 of 608.1 µg/mL for CNFs and 581.9 µg/mL for AgNPs at 24 h) and similar proliferative HaCaT cell activity. However, both nanomaterials showed very different results in the expression of thirteen genes (superoxide dismutase 1 (SOD1), catalase (CAT), matrix metallopeptidase 1 (MMP1), transforming growth factor beta 1 (TGFB1), glutathione peroxidase 1 (GPX1), fibronectin 1 (FN1), hyaluronan synthase 2 (HAS2), laminin subunit beta 1 (LAMB1), lumican (LUM), cadherin 1 CDH1, collagen type IV alpha (COL4A1), fibrillin (FBN), and versican (VCAN)) treated with the lowest non-cytotoxic concentrations in the HaCaT cells after 24 h. The AgNPs were capable of up-regulating only two genes (SOD1 and MMP1) while the CNFs were very effective in up-regulating eight genes (FN1, MMP1, CAT, CDH1, COL4A1, FBN, GPX1, and TGFB1) involved in the defense mechanisms against oxidative stress and maintaining and repairing tissues by regulating cell adhesion, migration, proliferation, differentiation, growth, morphogenesis, and tissue development. These results demonstrate CNF nanomaterials’ unique great potential in biomedical applications such as tissue engineering and wound healing.
Author(s): Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Ángel
Biomedicines
Published: 2021 Sep 3
DOI: 10.3390/biomedicines9091155
CDMF
The CDMF, hosted at the Federal University of São Carlos (UFSCar), is one of the Research, Innovation and Dissemination Centers (RIDC) supported by the São Paulo State Research Support Foundation (Fapesp), and also receives investment from the National Council Scientific and Technological Development (CNPq), from the National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).