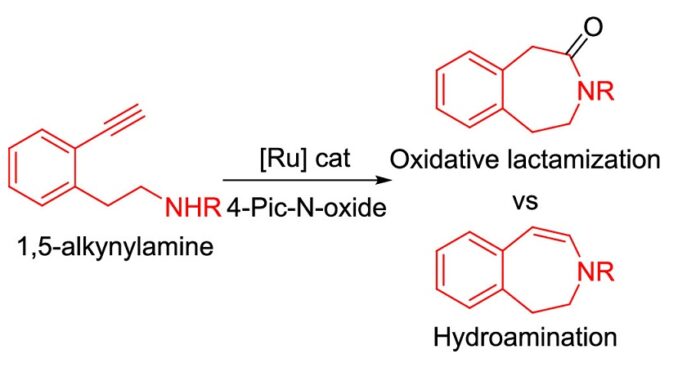
Chemoselective Ru-Catalyzed Oxidative Lactamization vs Hydroamination of Alkynylamines: Insights from Experimental and Density Functional Theory Studies
Abstract: The Ru-catalyzed intramolecular oxidative amidation (lactamization) of aromatic alkynylamines with 4-picoline N-oxide as an external oxidant has been developed. This chemoselective process is very efficient to achieve medium-sized ε- and ζ-lactams (seven- and eight-membered rings) but not for the formation of common δ-lactams (six-membered rings). DFT studies unveiled the capital role of the chain length between the amine and the alkyne functionalities: the longer the connector, the more favored the lactamization process vs hydroamination.
Author(s): Alvarez-Constantino, A. M.; Alvarez-Perez, A.; Varela, J. A.; Sciortino, G.; Ujaque, G.; Saa, C.
Journal of Organic Chemistry
Publication Date:December 29, 2022
DOI: https://doi.org/10.1021/acs.joc.2c02770
CDMF
The CDMF, hosted at the Federal University of São Carlos (UFSCar), is one of the Research, Innovation and Dissemination Centers (RIDC) supported by the São Paulo State Research Support Foundation (Fapesp), and also receives investment from the National Council Scientific and Technological Development (CNPq), from the National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).