Non-isothermal decomposition kinetics of conductive polyaniline and its derivatives
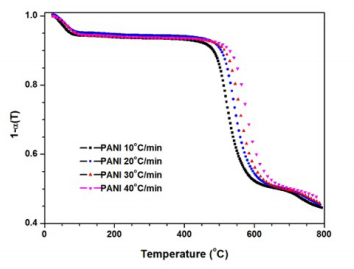
Abstract: The non-isothermal decomposition kinetics of conductive polyaniline (PANI) and its derivatives, poly(o-methoxyaniline) (POMA) and poly(o-ethoxyaniline) (POEA), was investigated by thermogravimetric analysis (TGA), under inert and oxidative atmospheres, using Flynn-Wall-Ozawa’s approach to assess the kinetic parameters of the decomposition process. The order of reaction was found to be dependent on the degree of conversion indicating that both the early and the later stages of polymer degradation were next the zero or pseudo zero order kinetics, whereas the intermediate stages follow a first order kinetics. The activation energy was found to be dependent on both the degree of conversion and PANI derivative. Activation energy values vary from 125 to 250 kJ/mol, to decompositions carried out under nitrogen, and 75 to 120 kJ/mol to oxidative atmosphere. Parent PANI presented the best thermal stability and suggesting that thermal stability is also influenced by derivatization and type of atmosphere used.
Author(s): Alves, WF; Malmonge, JA; Mattoso, LHC; de Medeiros, ES
POLIMEROS-CIENCIA E TECNOLOGIA
Volume: 28 Pages: 285-292 Published: AUG-SEP 2018
PDF: Non-isothermal decomposition kinetics of conductive polyaniline and its derivatives
DOI: 10.1590/0104-1428.03116