Synthesis of Lamellar Structures of Magnesium (II), Aluminum (III) and Iron (III) Hydroxides Interchanged with Carbonate Ion through Precipitation in pH 11
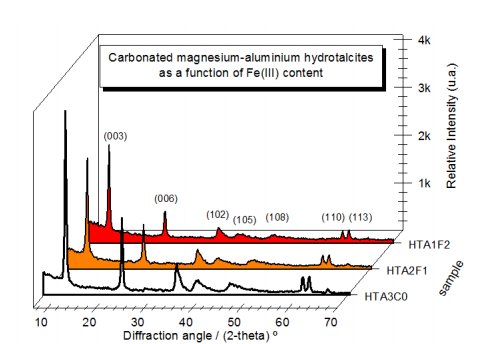
Abstract: The synthesis of layered double hydroxides has been investigated aiming innumerous applications, mainly as adsorbent, catalyst and catalyst support materials, due their ability to adsorb anionic species and several aqueous soluble compounds. The carbonated magnesium-aluminium hydrotalcites are known as the main class of the layered double hydroxides and new composition are often characterized under the view point of thermal stability, crystallinity and catalytic performance for many reactions. Few trivalent cations are able to replace the aluminum one due the severe restriction for oxidation state and ionic radii, but the iron (III) one seems to be high potential to improve some of the characteristics required for that materials, such as specificity for built-rebuilt bonds in organic molecules. In this work, we have synthesized carbonated magnesium-aluminum hydrotalcite samples through the coprecipitation at pH 11 and investigate the Fe(III) insertion at 10 and 20 mol%. Thermal analysis, FTIR spectrometry and X-ray diffractometry techniques were used to understand the influence of the Fe(III) co-substitution, keeping the Mg(II) molar fraction invariable among the samples. We show the iron (III) insertion affects the dehydration and dehydroxylation processes due the changes in M-OH bond energies Very homogeneous structures were obtained for all of the samples dried at 100 degrees C and a consistent lattice volume expansion was observed as a function of iron (III) content, which can be required for catalyst or catalyst matrix applications.
Author(s): Barbosa, GV; Meirelles, JS; de Oliveira, LCS; Amoresi, RAC; Zaghete, MA; Lima, SM; Cavalheiro, AA; da Silva, RCD
ORBITAL-THE ELECTRONIC JOURNAL OF CHEMISTRY
Volume: 10 Pages: 54-59 Published: JAN 2018
DOI: 10.17807/orbital.v10i1.1036