Towards high-efficient chalcopyrite photocathodes for water splitting: the use of cocatalysts beyond Pt
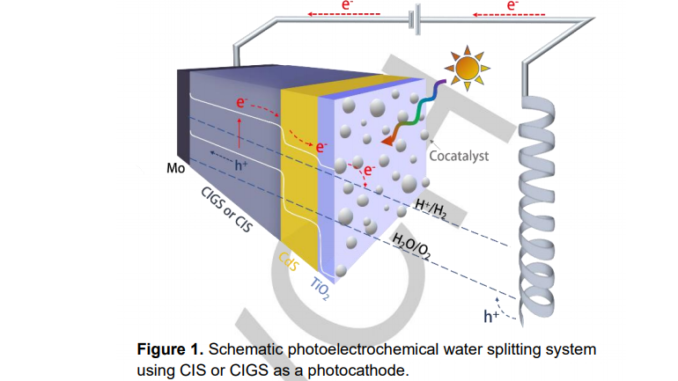
Abstract: Solar radiation is a renewable and clean energy source used in photoelectrochemical cells (PEC) to produce hydrogen gas as a powerful alternative to carbon-based fuels. Semiconductors play a vital role in this approach, absorbing the incident solar photons and converting them into electrons and holes. The hydrogen evolution reaction (HER) occurs in the interface of the p-type semiconductor that works as a photocathode in the PEC. Cu-chalcopyrite such as Cu(In, Ga)(Se,S)2 (CIGS) and CuIn(Se,S)2 (CIS) presents excellent semiconductor characteristics for this purpose but drawbacks as charge recombination, deficient chemical stability, and slow charge transfer kinetics, demands improvements like the use of n-type buffer layer, a protective layer, and a cocatalyst material. Concerning the last one, platinum (Pt) is the most efficient and stable material but the high price due to its scarcity imposes the search for inexpensive and abundant alternative cocatalyst. The present review highlights the use of metal alloys, transition metal chalcogenides, and inorganic carbon-based nanostructures as efficient alternative cocatalysts for HER in PEC.
Author(s): Salomao, A.C.; Araujo, M.S.; Santos, H.L.S.; Medina, M.; Mascaro, L.H.; Andrade Junior, M.A.S.
ChemSusChem
Published: 19 August 2021