Writers: Rabelo, Adriano C; Rosario, Adriane V; Trapp, Marília A; Filho, Edson Rodrigues; Forim, Moacir; Pereira, Ernesto C
Keywords: Catalytic; Methyl; Orange; Platinum; TiO2
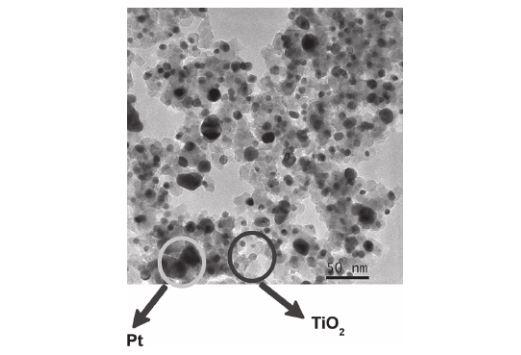
Abstract: A binary system composed of Pt clusters supported on TiO2 nanoparticles was prepared by Pechini Method and the samples were used in the catalytic wet oxidation of methyl orange. Transmission electron micrographs and X-ray diffraction patterns confirmed the composite formation. Liquid chromatography coupled with mass spectroscopy demonstrated that both nanoparticles, Pt and Pt–TiO2, led to the same reaction products. UV-Vis spectrum of individual reaction products indicate that azo group was attacked and the resonance between rings was broken. We have observed also an important increase in the kinect constant for the reaction of the Pt–TiO2samples. Normalizing the kinect constants by both surface area and mass, the values obtained for Pt–TiO2 sample is 12 times faster than those obtained for Pt nanoparticles.