Writers: La Porta, FA; Nogueira, AE; Gracia, L; Pereira, WS; Botelho, G; Mulinari, TA; Andres, J; Longo, E
Keywords: ZnS; Nanoparticles; Microwave-assisted solvothermal method; DFT; Photocatalysis; Photoluminescence
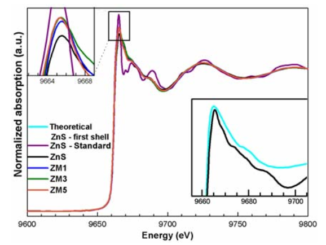
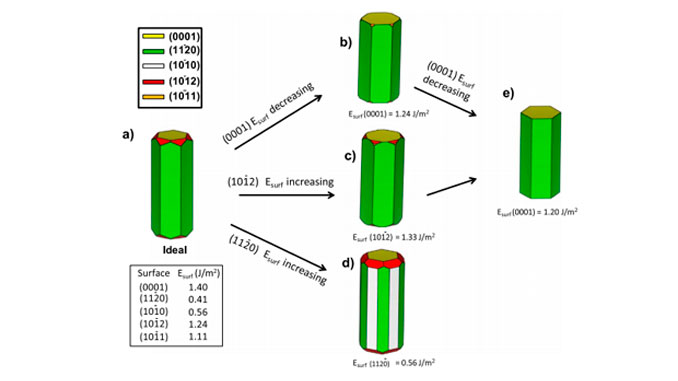
Abstract: From the viewpoints of materials chemistry and physical chemistry, crystal structure directly determines the electronic structure and furthermore their optical and photocatalytic properties. Zinc sulfide (ZnS) nanoparticles (NPs) with tunable photoluminescence (PL) emission and high photocatalytic activity have been obtained by means of a microwave-assisted solvothermal (MAS) method using different precursors (i.e., zinc nitrate (ZN), zinc chloride (ZC), or zinc acetate (ZA)). The morphologies, optical properties, and electronic structures of the as-synthesized ZnS NPs were characterized by X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), Brunauer Emmett Teller (BET) isotherms for N-2 adsorption/desorption processes, diffuse reflectance spectroscopy (DRS), PL measurements and theoretical calculations. Density functional theory calculations were used to determine the geometries and electronic properties of bulk wurtzite (WZ) ZnS NPs and their (0001), (100), (1120), (1011), and (1012) surfaces. The dependence of the PL emission behavior of ZnS NPs on the precursor was elucidated by examining the energy band structure and density of states. The method for degradation of Rhodamine B (RhB) was used as a probe reaction to investigate the photocatalytic activity of the as-Synthesised ZnS NPs under UV light irradiation. The PL behavior as well as photocatalytic activities of ZnS NPs were attributed to specific features of the structural and electronic structures. Increased photocatalytic degradation was observed for samples synthesized using different precursors in the following order: ZA < ZC < ZN. These results indicated that samples synthesized with ZN present a greater percentage of exposed (0001) surface than those synthesized with the ZC and ZA. Furthermore, the possible photodegradation mechanism of the as-prepared ZnS NPs were also briefly discussed.
DOI: 10.1016/j.jpcs.2016.12.025