CaSnO 3 obtained by modified Pechini method applied in the photocatalytic degradation of an azo dye
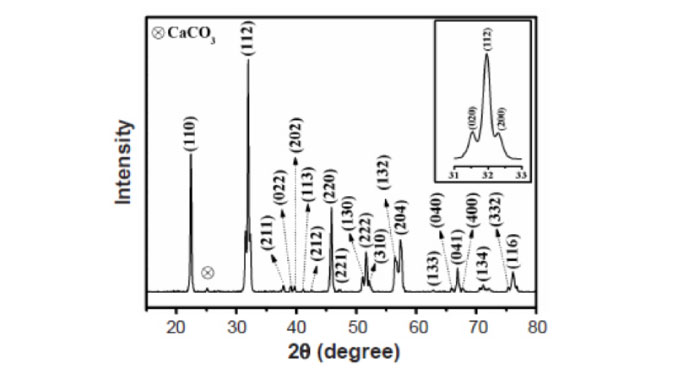
Abstract: Pure forms of alkaline-earth stannates with perovskite structure (ASnO3, A= Ca2+, Sr2+, Ba2+) have been used as photocatalysts. In this work, CaSnO3 perovskite sample was synthesized by a modified Pechini method at 800 ºC and characterized by X-ray diffraction (XRD), UV-visible spectroscopy, infrared spectroscopy and Raman spectroscopy. The photocatalytic degradation of remazol golden yellow (RNL) dye under UV radiation was evaluated. The XRD pattern showed that the synthesis method favored the orthorhombic CaSnO3 crystallization. According to the Raman spectrum, a material with high short-range order was obtained despite of the relatively low synthesis temperature, compared to the solid-state reaction one. The highest photocatalytic activity was attained at pH 3, which presented 51% discoloration and improved activity of 35% compared to discoloration solely due to adsorption (absence of radiation). The point of zero charge (PZC) and the photocatalytic results indicated that a direct mechanism prevailed at pH 3, whereas an indirect mechanism prevailed at pH 6.
Author(s): Lucena, G. L.; Lima, L. C. de; Honório, L. M. C.; Oliveira, A. L. M. de; Tranquilim, R. L.; Longo, E. ; Souza, A. G. de; Maia, A. da S.; Santos, I. M. G. dos.
Cerâmica
Volume: 63 | Ed: 368 | Pages: 536-541 | Published: 2017-12
PDF: CaSnO 3 obtained by modified Pechini method applied in the photocatalytic degradation of an azo dye
DOI: 10.1590/0366-69132017633682190




