Synthesis of BiVO4 via oxidant peroxo-method: insights into the photocatalytic performance and degradation mechanism of pollutants
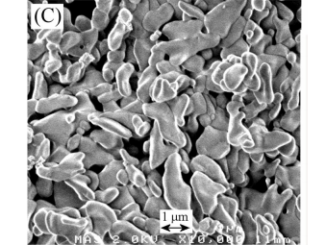
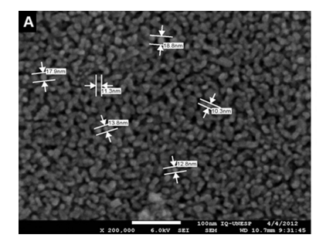
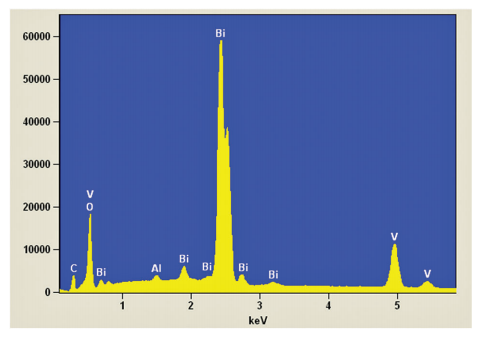
Abstract: This paper reports the synthesis of monoclinic bismuth vanadate (BiVO4) by the oxidant peroxide method with crystallization under hydrothermal conditions, and its catalytic performance on the photodegradation of pollutants under visible light. The as-synthesized BiVO4 materials were characterized by means of X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS), Raman spectroscopy, Ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) and scanning electron microscopy (SEM). Hydrothermal treatment above 80 degrees C was required to obtain pure monoclinic BiVO4 phase by releasing V5+ ions from vanadium peroxo complexes. With the increase in hydrothermal reaction temperature, the particle size decreased. All BiVO4 samples presented large size and shape distribution and band gap of approximately 2.40 eV. The as-prepared BiVO4 catalysts showed high photoactivity for decomposition of model pollutants, methylene blue (MB) and rhodamine B (RhB) dyes, under exposure to visible light. The photodegradation mechanism was evaluated by adding scavengers, DMSO and KBrO3, which were used to probe (OH)-O-center dot radical and conduction band (CB) electrons, respectively. It was observed that photodegradation of MB and RhB dyes is caused by the action of (OH)-O-center dot radicals, and that BiVO4 CB electrons do not have reduction potential sufficiently high to reduce dissolved oxygen to O-2(-center dot). It was proven that the indirect mechanism, i.e. (OH)-O-center dot radical formation, plays the major role on the BiVO4-assisted photodegradation process.
Author(s): Lopes, OF; Carvalho, KTG; Macedo, GK; de Mendonca, VR; Avansi, W; Ribeiro, C
NEW JOURNAL OF CHEMISTRY
Volume: 39 Pages: 6231-6237 Published: 2015
DOI: 10.1039/c5nj00984g