Gold nanoparticles supported on magnesium ferrite and magnesium oxide for the selective oxidation of benzyl alcohol
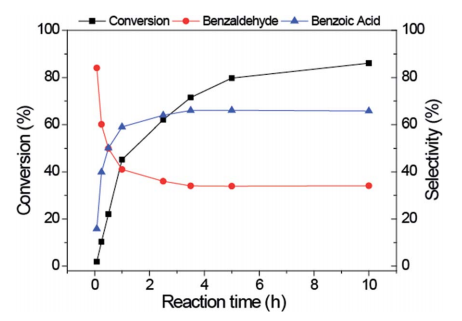
Abstract: Au nanoparticles (Au NPs) have gained significant attention as catalysts for the selective oxidation of alcohols; however, the catalytic activity is highly dependent on the presence of a base. Alternatives to the use of strong bases, such as NaOH, are still needed. Here, we explored the basicity of magnesium ferrite/oxide supports to study the catalytic behaviour of supported Au NPs for the oxidation of benzyl alcohol. The presence of Mg2+ ions in the ferrite structure improved the catalytic activity of supported Au NPs to ca. 35% conversion in the absence of an additional base. After modifying the support with MgO, the catalytic activity of supported Au NPs was further improved to ca. 50% conversion, but the catalyst deactivated in successive recycling tests. When the catalysts were tested in the presence of a sub-stoichiometric amount of K2CO3, they became more active and remained stable upon recycling with no loss of activity and selectivity for the preferential production of benzoic acid.
Author(s): de Moura, EM; Garcia, MAS; Goncalves, RV; Kiyohara, PK; Jardim, RF; Rossi, LM
RSC ADVANCES
Volume: 5 Pages: 15035-15041 Published: 2015
DOI: 10.1039/c4ra16159a