Researchers obtain promising results against capacity loss in vanadium batteries
Chem Europe
An article by researchers at the Center for Development of Functional Materials (CDMF) in Brazil describes a successful strategy to mitigate charge capacity loss in vanadium redox flow batteries, which are used by electric power utilities among other industries and can accumulate large amounts of energy. CDMF is a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by the Federal University of São Carlos (UFSCar) in São Paulo state.
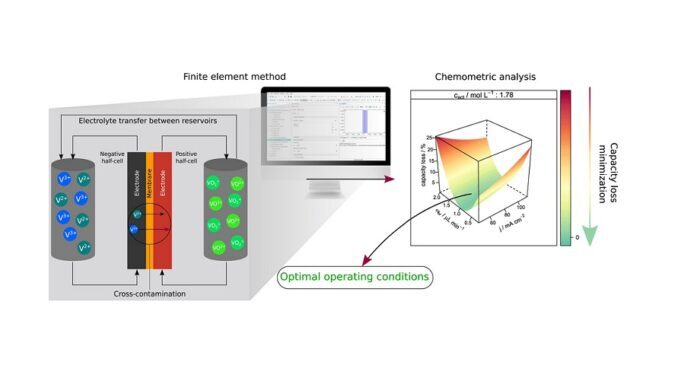
The study involved computer simulations designed to find out how ion leakage between the anolyte and catholyte, called transport loss, leads to battery deactivation, and how to mitigate this loss so as to keep ion concentration constant over time. Initially, the researchers estimated the effects of current density, active species concentration and volumetric flow on capacity loss. The second stage sought optimal conditions to minimize capacity loss based on the flow between electrolyte tanks in the opposite direction to cross-contamination (transport of electroactive species through the membrane).
The results showed current density and active species concentration to be the main variables affecting capacity loss. According to the researchers, their approach successfully mitigated cross-contamination in different combinations of the two variables, providing an optimal flow between electrolyte tanks under different operating conditions.
Ernesto Pereira, last author of the article and a professor at UFSCar, noted that the main advantage of redox flow batteries is lack of electrode aging as the electroactive components are dissolved in solutions instead of being coated onto electrodes.
Commercial vanadium redox flow batteries are expected to have a longer lifetime than other types, although the study was conducted on a small scale. “Energy efficiency loss due to aging is minimal, given the slow pace of aging,” he said.
The researchers explained that they are exploring and analyzing flow batteries computationally, with commercial batteries as a model, as part of a broader strategy that includes the development of novel organic substances for this type of battery.
