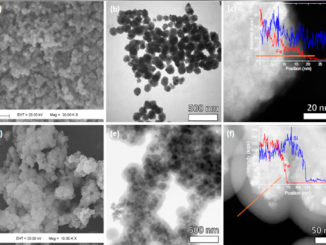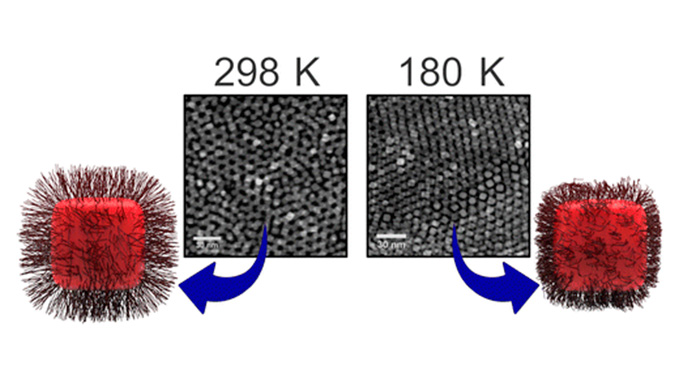
Writers: Marco A. L. Cordeiro, Edson R. Leite, and Eric A. Stach
Keywords: Nanoparticle; Structure
Abstract: The tailoring of nanoparticle superlattices is fundamental to the design of novel nanostructured materials and devices. To obtain specific collective properties of these nanoparticle superlattices, reliable protocols for their self-assembly are required. This study provides insight into the self-assembly process by using oleate-covered CeO2 nanoparticles (cubic and polyhedral shapes) through the correlation of experimental and theoretical investigations. The self-assembly of CeO2 nanoparticles is controlled by tuning the colloid deposition parameters (temperature and evaporation rate), and the ordered structures so obtained were correlated to the Gibbs free energy variation of the system. The analysis of the interparticle force contributions for each structure showed the importance of both the effective ligand mean size and its Flory–Huggins parameter in determining the total potential energies. Additionally, the roles of ligand solubility and effective mean size were used to understand the formation of specific superlattice phases as a function of temperature and ligand accommodation in the arrangement. Furthermore, the face-to-face interactions between nanoparticles were correlated to the type of exposed crystallographic facet in each particle.