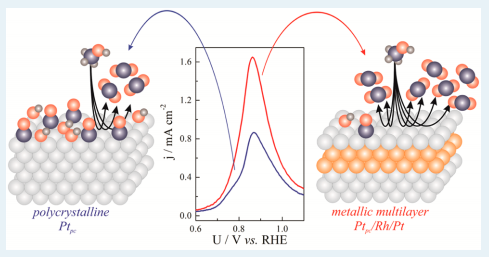
Oscillatory Electro-oxidation of Methanol on Nanoarchitectured Pt-pc/Rh/Pt Metallic Multilayer
Abstract: The oscillatory electro-oxidation of methanol was studied on polycrystalline Pt-pc and Pt-pc/Rh-2.0/Pt-1.0 metallic multilayers. The surfaces investigated consisted of 1.0 Pt outlayer surface deposited onto 2.0 Rh intralayers beneath a Pt outlayer and over the polycrystalline Pt-pc, substrate. In addition to experimental studies, numerical simulations were performed using a dimensionless kinetic model for the electro-oxidation of methanol in order to provide a better understanding of the role 0 played by the nanostructured metallic multilayer electrode in the electrocatalytic activity. A comparable electrochemical behavior found for cyclic voltammetry in blank acidic media was observed in both electrodes. Remarkably, an increase of 90% in the peak current density around 0.88 V vs. RHE in the electro-oxidation of methanol appeared when Pt-pc/Rh-2.0/Pt-1.0 was utilized. The numerical simulations suggested that this increase in the electrocatalytic activity for the metallic multilayer electrode is due to the prevention of carbon monoxide adsorption on the surface and a consequent increase in the production of carbon dioxide from the direct pathway. Indeed, a decrease in the reaction rate constant of carbon monoxide formation resulted in an increase of the current density associated with CO2 formation in the potentiodynamic sweep, in addition to the decrease in amplitude and frequency of the oscillatory time series. As the rate of carbon monoxide adsorption is suppressed by the presence of the metallic multilayers, the intrinsic drift usually found in the oscillatory electro-oxidation of methanol was enhanced and oscillations ceased earlier. Overall, the combination of electrochemical experiments and numerical simulations suggests that carbon monoxide acts as a poisoning species instead of a reaction intermediate in the electro-oxidation of methanol.
Author(s): Nagao, R; Freitas, RG; Silva, CD; Varela, H; Pereira, EC
ACS CATALYSIS
Volume: 5 Pages: 1045-1052 Published: FEB 2015
PDF: Oscillatory Electro-oxidation of Methanol on Nanoarchitectured Pt-pcRhPt Metallic Multilayer
DOI: 10.1021/cs501652u