Insights on the mechanism of solid state reaction between TiO2 and BaCO3 to produce BaTiO3 powders: The role of calcination, milling, and mixing solvent
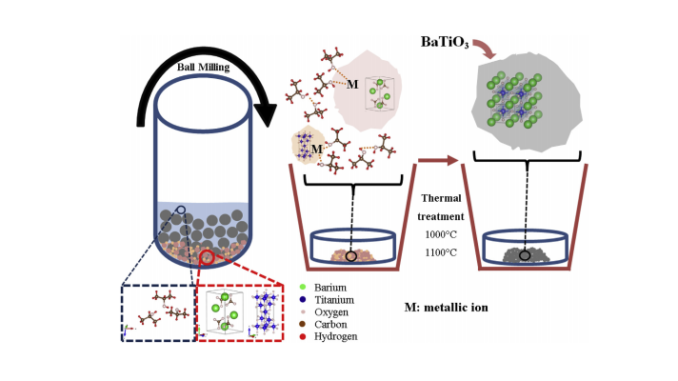
Abstract: Barium titanate (BaTiO3) is well-known for its variety of macroscopic properties, however, the mechanism of formation of this oxide in microscale remains poorly understood. We prepared the BaTiO3 nanoparticles and studied them as a function of milling time and temperature. A systematic study was performed to understand the reaction mechanism of undissociated and dissociated 2-propanol molecules on TiO2 and BaCO3 particles. Structural evolution and thermal decomposition of surface particles were carried out and studied by X-ray diffraction, scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy analysis. Structural parameters refined by Rietveld analysis using X-ray diffraction data revealed a tetragonal structure with P4/mm space group, which was due to the dependence of functional groups on the milling time and thermal treatment. The divergence of tetragonality as a function of thermal treatment was identified for particles of size <400 nm, which probably happened due to surface defects which decreased in low temperature of about 1000 °C. Particle sizes precursors decreased as a function of the milling time from 18 μm (2 h milling) to 1.2 μm (24 h of milling). While by increasing the thermal treatment of samples from 1000 °C to 1100 °C the particles size increased, respectively, 0.24 μm and 0.36 μm. The effect of the particle size of TiO2 plays an important role in the reaction kinetics of the formation of BaTiO3. Our results suggest that the reaction proceeds through a mechanism in which the intermediate processes of TiO2, BaCO3 and dissociation of BaCO3 are trapped by the dissociated and undissociated 2-propanol molecules, which resulted in depletion of 2-propanol-related functional groups in the BaTiO3 particle surface at higher temperature.
Author(s): Clabel HJ.L.; Awan, I.T.; Pinto, A.H.; Nogueira I.C.; Clabel H, V.D.N.Bezzon; Leite, E.R.; Balogh, D.T.; Mastelaro, V.R.; Ferreira, S.O.; Marega Jr, E.
Ceramics International
Volume 46, Issue 3, Published 15 February 2020, Pages 2987-3001