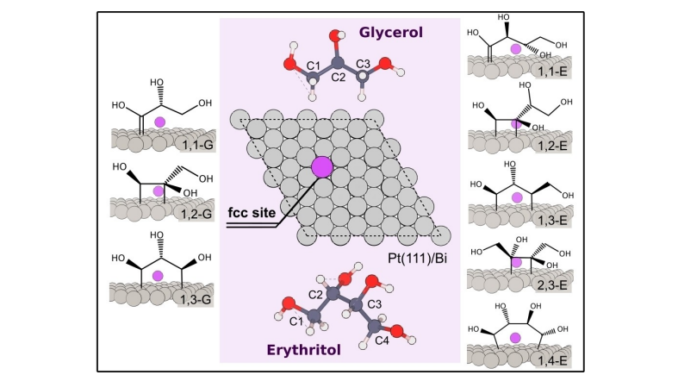
Electro-Oxidation of Polyols on Bi-Modified Pt in Acidic Media (HClO4). Understanding Activity and Selectivity Trends
Abstract: Herein we show that Pt(111) and Pt(100) can produce the ketone through the oxidation of the secondary carbon of the polyols. After the Bi modification, the selectivity for the ketone formation increases. On the other hand, we observe that pure and Bi-modified Pt(110) only produced the C3 molecules oxidized in the primary carbon, and it is the only facet that shows an enhancement in the activity due to the modification. In line with these findings, small Pt nanoparticles are not selective for ketone formation. Finally, based on data obtained through DFT calculations, we suggest that positively charged Bi adatoms interact with the OH- groups of the enediol-like intermediate (believed to be the precursor for the ketone/aldehyde production), facilitating the oxidation of the secondary carbon to produce DHA.
Author(s): Gabriela Soffiati, Victor Y. Yukuhiro, Swathi P. Raju, Dr. Matheus B. C. De Souza, Leonardo Marquezini, Dr. Edison Z. da Silva, Dr. Pablo S. Fernández, Dr. Miguel A. San-Miguel
ChemCatChem
First published: 08 March 2023
DOI: https://doi.org/10.1002/cctc.202300252
CDMF
The CDMF, hosted at the Federal University of São Carlos (UFSCar), is one of the Research, Innovation and Dissemination Centers (RIDC) supported by the São Paulo State Research Support Foundation (Fapesp), and also receives investment from the National Council Scientific and Technological Development (CNPq), from the National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).