Writers: Sergio A. S. Farias. E. Longo, R. Gargano, João B. L. Martins
Keywords: CO2 adsorption; Electronic localization function; First principles; Plane wave; ZnO
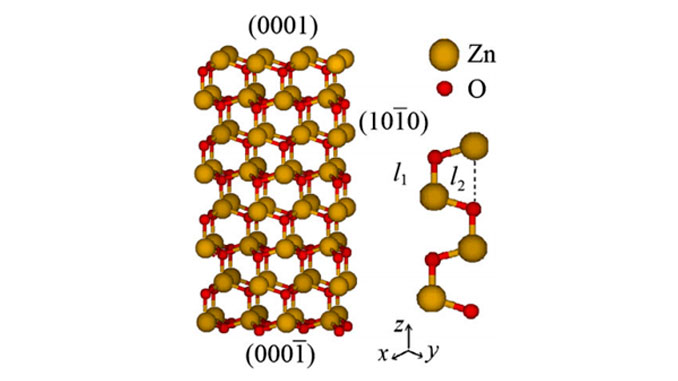
Abstract: Physical and chemical adsorption of CO2 on ZnO surfaces were studied by means of two different implementations of periodic density functional theory. Adsorption energies were computed and compared to values in the literature. In particular, it was found that the calculated equilibrium structure and internuclear distances are in agreement with previous work. CO2 adsorption was analyzed by inspection of the density of states and electron localization function. Valence bands, band gap and final states of adsorbed CO2 were investigated and the effect of atomic displacements analyzed. The partial density of states (PDOS) of chemical adsorption of CO2 on the ZnO(0001) surface show that the p orbitals of CO2 were mixed with the ZnO valence band state appearing at the top of the valence band and in regions of low-energy conduction band.
See PDF: CO2 adsorption on polar surfaces of ZnO
DOI: 10.1007/s00894-012-1636-4