Writers: R. L. Grosso, J. R. Matos, E. N. S. Muccillo
Keywords: Zirconia; Scandium; Dysprosium; TG–DTA; FTIR; DRX
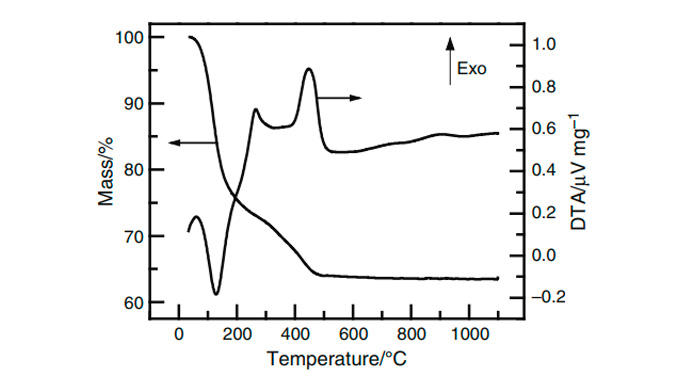
Abstract: Zirconia containing 10 mol% scandia and x mol% dysprosia (0 ≤ x ≤ 1.5) gels was synthesized by simultaneous precipitation at room temperature. The aim of this work is to verify the effect of dysprosium on the cubic phase stabilization of the zirconia–scandia solid electrolyte. The gel was characterized by thermogravimetry, differential scanning calorimetry, and differential thermal analyses. The thermally treated powders were analyzed by Fourier transform infrared spectroscopy, thermal analyses, and X-ray diffraction techniques. For comparison purpose, a commercial zirconia–10 mol% scandia powder was subjected to some characterization techniques. The infrared spectrum shows characteristic absorption bands due to residual material from the synthesis on the surface of the powder particles. Nanostructured powders were obtained after thermal treatments at 500 °C for 2 h. Infrared spectroscopy and X-ray diffraction results evidence the stabilization of the cubic phase in zirconia–scandia containing dysprosium. The thermal stability of the cubic phase during thermal cycling was ascertained by thermal analysis.