Writers: Souza, J. S. and Carvalho, W. M. and Souza, F. L. and Ponce-de-Leon, C. and Bavykin, D. V. and Alves, W. A.
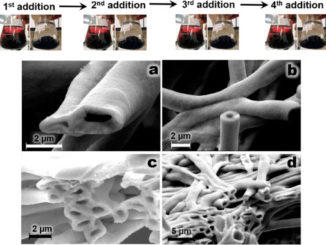
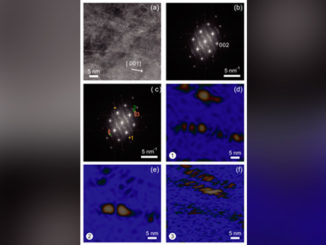
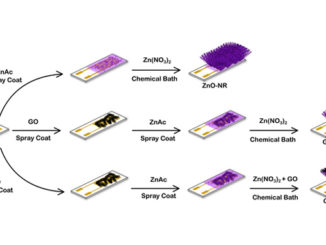
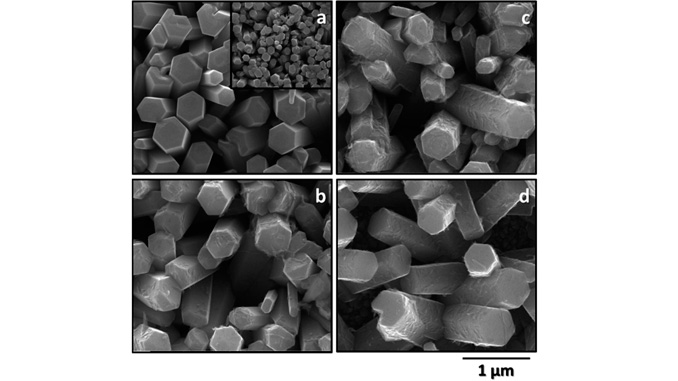
Abstract: Studies involving water splitting to form hydrogen and oxygen have attracted attention because H2 is considered the fuel of the future. Photoelectrocatalysts have been widely used for this application{,} and several metal oxides can be applied as catalysts. Among them{,} we highlight zinc oxide nanorods (ZnONRs) and titanate nanotubes (TiNTs); however{,} their individual nanostructures exhibit disadvantages. For example{,} ZnONRs show rapid recombination of the photogenerated charges{,} and TiNTs give rise to randomly oriented films; these disadvantages limit their application as photoanodes. In this study{,} for the first time{,} we present a new class of multihierarchical electrodes based on TiNT-decorated ZnONR films that exhibited superior results to the individual species. The TiNTs are homogenously dispersed over the surface of the rods without forming agglomerates{,} giving rise to a heterojunction that exhibits lower recombination rates. It was found that the results are better when the contents of TiNTs in the electrode are higher; thus{,} glycine was successfully used as a bridge to link both of the structures{,} increasing the amount of TiNTs decorating the rods. As a result{,} the photocurrent generated with these multihierarchical electrodes is higher than that obtained with pure ZnONR electrodes (0.9 mA cm-2 and 0.45 mA cm-2 at 1.23 VRHE{,} respectively){,} and the electrode potentials for O2 evolution are lower than those observed for pure TiNT electrodes (0 V and 0.8 V vs. ERHE{,} respectively). The IPCE values are also higher for the multihierarchical electrodes.