Coupled electronic and morphologic changes in graphene oxide upon electrochemical reduction
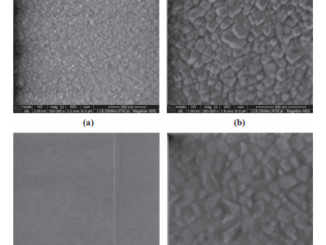
Abstract: A systematic study of the electrochemical reduction of graphene oxide was performed. The graphene oxide was reduced electrochemically in phosphate buffer solution by applying potential of -0.8 V for different times. The graphene oxide and the electrochemically reduced graphene oxide were characterized using ex-situ field-emission gun scanning electron microscopy, in-situ Raman scattering and in-situ atomic force microscopy. Raman scattering showed that the reduction process is accompanied by an increase in the ratio between the D and the G bands of graphene, while the microscopies analyses revealed that the reduction procedure promotes changes in the morphology of the graphene oxide sheets that lead to an increase of the system roughness. Electrochemical impedance spectroscopy showed that reduction of graphene oxide promotes a decrease of the charge-transfer resistance upon electrochemical reduction. This observation was in agreement with the changes observed using cyclic voltammetry, which showed a reduction process improve the reversibility and increase the current peak. The increase in the roughness and an improvement of the electronic mobility brought upon electrochemical reduction are a function of the increase in the edge plane-like defects in the graphene layers. (C) 2015 Elsevier Ltd. All rights reserved.
Author(s): Moraes, FC; Freitas, RG; Pereira, R; Gorup, LF; Cuesta, A; Pereira, EC
CARBON
Volume: 91 Pages: 11-19 Published: SEP 2015
PDF: Coupled electronic and morphologic changes in graphene oxide upon electrochemical reduction
DOI: 10.1016/j.carbon.2015.04.038