A comparison of charge-transfer mechanisms at rotated disk electrode for biomimetic binuclear and tetranuclear oxo-manganese complex in aqueous solution
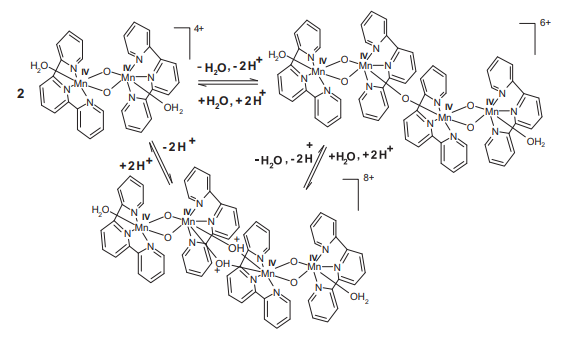
Abstract: Many high-valence multinuclear mu-oxo-bridged manganese complexes have been synthesized to mimic the active site of the natural enzymes. The electrochemical and kinetic parameters were determined for two mimicking complexes ([(Mn2O2)-O-IV(terpy)(2)(H2O)(2)](4+) and [(Mn4O5)-O-IV(terpy)(4)(H2O)(2)](6+)) by cyclic voltammetry and linear sweep voltammetry using a rotating disk electrode (RDE). Stability and kinetic behavior are directly related to mu-oxo bridges, where the mu-oxo-bridge provides a fast electron transition between metal centers due to stabilization of dx(2) – y(2) orbitals by the oxygen bond. On the other hand, when the dx(2) – y(2) orbitals are stabilized by aqua ligands by a coordination bond, a displacement of oxidation potential to more positive potential was observed. A shift of the potential to more negative values with increase in rotation rate was observed, which can be ascribed to a chemical step. The chemical step involves the dimerization process of the binuclear oxo-manganese complex to tetranuclear oxo-manganeses complex. (C) 2014 Elsevier B.V. All rights reserved.
Author(s): Martin, CS; Teixeira, MFS
INORGANICA CHIMICA ACTA
Volume: 425 Pages: 76-82 Published: JAN 30 2015
DOI: 10.1016/j.ica.2014.10.009