Insights into formation of anatase TiO2 nanoparticles from peroxo titanium complex degradation under microwave-assisted hydrothermal treatment
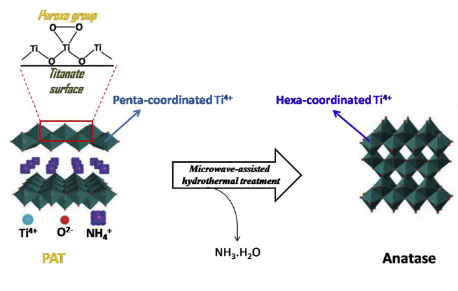
Abstract: Several methods for the synthesis of anatase TiO2 have been reported in the literature. In particular, the microwave-assisted hydrothermal crystallization of Ti-peroxocomplexes has enabled the phase and morphological control of TiO2 nanoparticles by adjusting the synthesis conditions, such as pH, time and temperature, with significant increase in the reaction rate compared to the conventional sol-gel techniques. In this paper, we uncover the mechanism involved in the formation of anatase TiO2 nanoparticles from the Ti-peroxo complex precursor route. Structural characterizations (XRD, Raman, TEM and XANES) revealed that the Ti-peroxo complex precursor exhibits a titanate-like structure, comprised of bulk NH4+ ions and surface peroxo groups. The NH4+ ions release during hydrothermal treatment is determining on the anatase TiO2 nanoparticle formation, which occurs concomitantly with the decomposition of peroxogroups. UV-induced dye photodegradation tests involving the Ti-peroxo complex precursor and TiO2 nanoparticles showed that the precursor is not able to form hydroxyl radicals that are the main chemical species responsible for the dye photodegradation, despite the presence of peroxo groups at its surface. The results herein highlight the mechanism involved in the formation of anatase TiO2 nanoparticles, which could assist in the development of new synthesis approaches based on Ti-peroxocomplex precursors.
Author(s): de Mendonca, VR ; Lopes, OF ; Avansi, W ; Arenal, R ; Ribeiro, C
CERAMICS INTERNATIONAL
Volume: 45 Edition: 17 Pages: 22998-23006 Parte: B Published: DEC 1 2019
DOI: 10.1016/j.ceramint.2019.07.345