One-step electrodeposited nickel phosphide electrode for pH-universal electrochemical hydrogen production
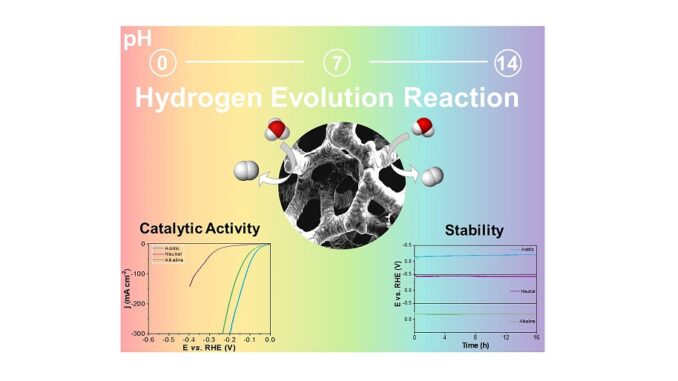
Abstract: Herein, we describe the development and study of the relationship between the Ni molar ratio of amorphous nickel phosphide (Ni-P) electrodes synthesized via electrodeposition on Ni foam (NF) applied as a non-precious electrocatalyst for the Hydrogen Evolution Reaction (HER) in a pH-universal. The 3-Ni-P electrode displayed outstanding HER performance in alkaline, neutral, and acidic conditions with an overpotential of 69, 165, and 56 mV at –10 mA cm–2. Furthermore, the Ni-P films showed excellent stability under the different conditions studied. The optimized 3-Ni-P electrode performance was attributed to its granular structure with a large surface area, enabling good interaction with the electrolyte, which endorses the HER kinetics. These results are relevant concerning an adequate choice of stable catalyst material, easy to synthesize and capable of operating in a wide range of pH with high efficiency for HER.
Author(s): A.B. Silva, M. Medina, L.A. Goulart, L.H. Mascaro
Electrochimica Acta
Published: 20 January 2024, Volume 475, 143679
DOI: https://doi.org/10.1016/j.electacta.2023.143679
CDMF
The CDMF, hosted at the Federal University of São Carlos (UFSCar), is one of the Research, Innovation and Dissemination Centers (RIDC) supported by the São Paulo State Research Support Foundation (Fapesp), and also receives investment from the National Council Scientific and Technological Development (CNPq), from the National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).