Macro- and Nano-Porous Ag Electrodes Enable Selective and Stable Aqueous CO2 Reduction
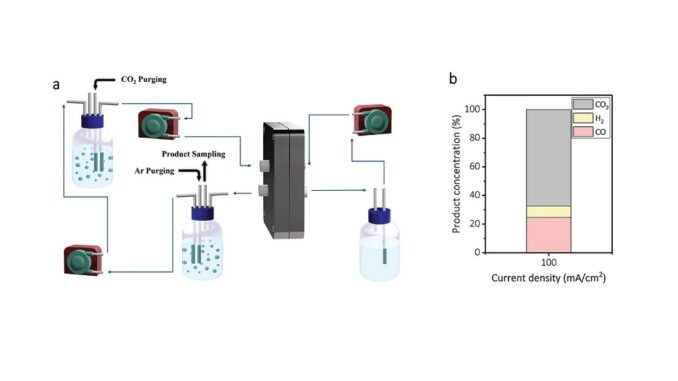
Abstract: Electrochemical carbon dioxide (CO2) reduction from aqueous solutions offers a promising strategy to overcome flooding and salt precipitation in gas diffusion electrodes used in gas-phase CO2 electrolysis. However, liquid-phase CO2 electrolysis often exhibits low CO2 reduction rates because of limited CO2 availability. Here, a macroporous Ag mesh is employed and activated to achieve selective CO2 conversion to CO with high rates from an aqueous bicarbonate solution. It is found that activation of Ag surface using oxidation/reduction cycles produces nanoporous surfaces that favor CO2-to-CO conversion. Notably, it is found that a combination of dissolved CO2 in bicarbonate solution with CO2 generated in situ from bicarbonate ions enables increased CO2 availability and a CO2-to-CO conversion rate over 100 mA cm−2. By optimizing the oxidation/reduction cycles to fine-tune the structure of Ag surface, CO2-to-CO conversion is reported from a bicarbonate solution with CO Faradaic efficiency of over 85% at current density of 100 mA cm−2, high concentration of 24.7% at outlet gas stream and stability of over 100 h with maintaining CO FE over 85% during whole reaction time.
Author(s): Behnam Nourmohammadi Khiarak, Gelson T. S. T. da Silva, Valentine Grange, Guorui Gao, Viktoria Golovanova, F. Pelayo de García de Arquer, Lucia H. Mascaro, Cao-Thang Dinh.
Small
Published: 23 December 2024
DOI: https://doi.org/10.1002/smll.202409669
CDMF
The CDMF, hosted at the Federal University of São Carlos (UFSCar), is one of the Research, Innovation and Dissemination Centers (RIDC) supported by the São Paulo State Research Support Foundation (Fapesp), and also receives investment from the National Council Scientific and Technological Development (CNPq), from the National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).