Writers: M.H.M.T. Assumpção; A. Moraes; R.F.B. De Souza; M.L. Calegaro; M.R.V. Lanza; E.R. Leite; M.A.L. Cordeiro; P. Hammer; M.C. Santos
Keywords: Oxygen reduction reaction; Electrogeneration of hydrogen peroxide; Cerium oxide nanoparticles
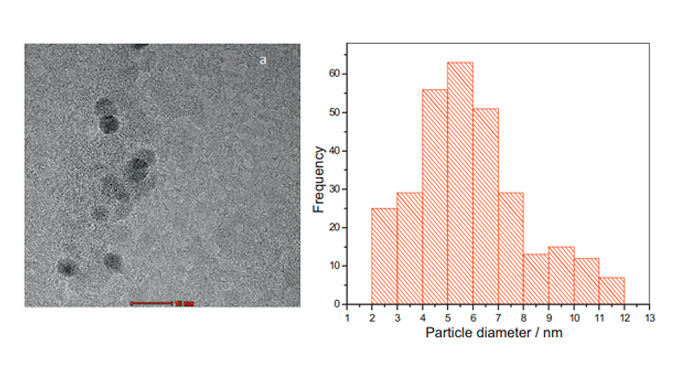
Abstract: This work describes the influence of the preparation method and the carbon support using a low content of cerium oxide nanoparticles (CeO2/C 4%) on H2O2 electrogeneration via the oxygen reduction reaction (ORR). For this purpose, the polymeric precursor (PPM) and sol–gel (SGM) methods with Vulcan XC 72R (V) and Printex L6 (P) supports were employed. The materials were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM). The XRD analysis identified two phases comprising CeO2 and CeO2−x. The smallest mean crystallite size was exhibited for the 4% CeO2/C_PPM_P material, which was estimated using the Debye–Scherrer equation to be 6 nm and 4 nm for the CeO2 and the CeO2−x phases, respectively, and was determined by TEM to be 5.9 nm. XPS analysis was utilized to compare the oxygen content of the 4% CeO2/C_PPM_P to Printex L6. The electrochemical analysis was accomplished using a rotating ring-disk electrode. The results showed that the 4% CeO2/C specimen, prepared by PPM and supported on Printex L6, was the best electrocatalyst for H2O2 production in 1 mol L−1 NaOH. This material showed the highest ring current, producing 88% H2O2 and transferring 2.2 electrons per O2 molecule via the ORR at the lowest onset potential. Additionally, the ring-current of the 4% CeO2/C_PPM_P material was higher than that of Vulcan XC 72R and Printex L6, the reference materials for H2O2 production, indicating the highest electrocatalytic activity for the 4% CeO2/C_PPM_P material.