Writers: M. R. D. Bomio, R. L. Tranquilin, F. V. Motta, C. A. Paskocimas, R. M. Nascimento, L. Gracia, J. Andres and E. Longo
Keywords: Photocatalytic; Combined Experimental; Theoretical Study
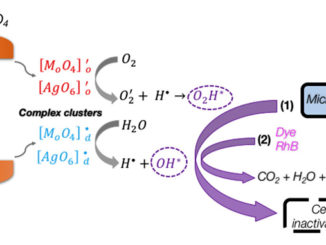
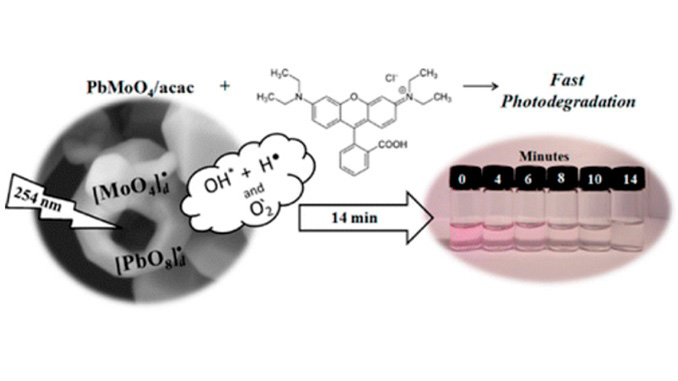
Abstract: A complementary combination of experimental work and first-principle calculations, based on the density functional theory (DFT) method, has been used to increase our limited understanding of the enhanced photocatalytic activity of PbMoO4 powders with predominant (111), (100), (011), and (110) facets. In this work, PbMoO4 powders were prepared by the coprecipitation method and processed on a hydrothermal reactor at 100 °C/10 min. The variation of different types of modifiers such as acetylacetone (acac) or polyvinylpyrrolidone (PVP) is found to play a crucial role in controlling the particle size and morphology of products and their photocatalytic properties. The structure and morphology of these crystals were characterized by X-ray diffraction (XRD), micro-Raman (MR) spectroscopy, field-emission gun scanning electron microscopy (FEG-SEM), and ultraviolet visible (UV–vis) absorption spectroscopy. Furthermore, the as-synthesized PbMoO4 micro-octahedrons without the presence of the (001) surface exhibit enhanced activity for the photodegradation of rhodamine B (RhB) under ultraviolet–visible light irradiation. On the basis of the theoretical and experimental results, we provide a complete assignment of the micro-Raman spectra of PbMoO4, while a growth mechanism for the formation of PbMoO4 micro-octahedrons was systematically discussed. A schematic illustration of the probable formation of morphologies in the whole of the synthetic process was also proposed, which reveals that the high photocatalytic activity is attributed to the absence of the (001) facet.