Writers: M.T.S. Tavares, L.X. Lovisa, V.D. Araújo, E. Longo, M.S. Li, R.M. Nascimento, C.A. Paskocimas, M.R.D. Bomio, F.V. Motta
Keywords: Indium hydroxide; Zinc; Doping; Microwave; Photocatalytic activity
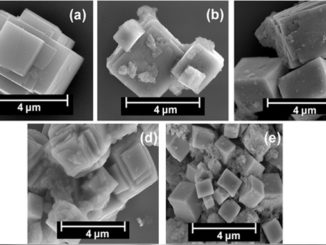
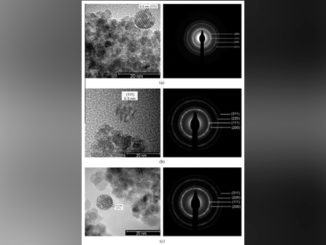
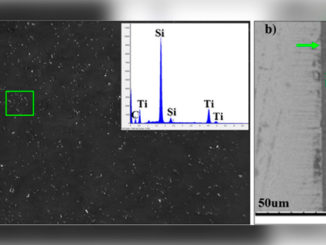
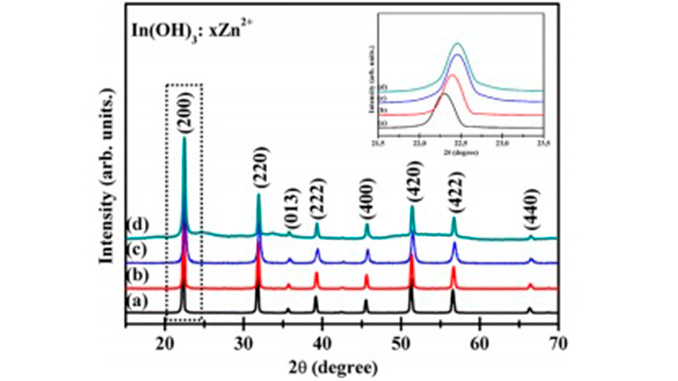
Abstract: Crystalline zinc-doped indium hydroxide structures were prepared by the rapid and efficient microwave-assisted hydrothermal (MAH) method. X-ray diffraction (XRD), field emission gun-scanning electron microscopy (FEG-SEM), photoluminescence (PL) and UV– visible (UV–vis) spectroscopy were used to characterization the materials. FEG-SEM images revealed that pure In(OH)3 samples and Zn-doped samples exhibit nanocubic morphologies with a wide range of particle sizes. Relative intensities of PL emissions decreased as the Zn ion concentration increased from 0 to 4 mol%. UV–vis spectra indicate that Zn ion doping caused a band gap decrease with increased doping of 0% to 4% Zn, respectively, from 5.15 eV for 4.96 eV. Samples with Zn in the crystal lattice showed better photocatalytic activity and degradation of the RhB dye after 16 min of UV exposure.