Writers: M. Piacenti-Silva, , E.S. Bronze-Uhle, J.V. Paulin, C.F.O. Graeff
Keywords: Melanin; Dimethyl sulfoxide; Synthesis; Temperature; Optimization; Photoabsorption
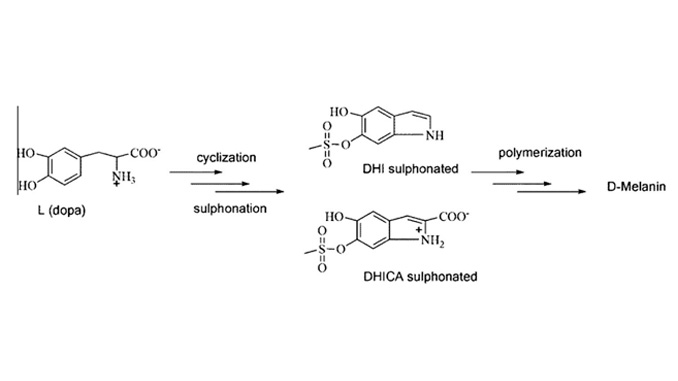
Abstract: Melanins are a class of pigmentary conjugated macromolecules found in many biological systems. Functionalization of synthetic melanin provides interesting new properties like the greater solubility of melanin synthesized in dimethyl sulfoxide, d-Melanin. In this work we have studied the influence of temperature on d-Melanin synthesis and its properties. To this end, UV–Vis, Fourier-transform infrared (FTIR) spectroscopy and Nuclear Magnetic Resonance (NMR) techniques have been employed to analyze d-Melanin synthesized within the range of 25–100 °C. Our results reveal that by increasing the synthesis temperature up to 100 °C, the synthesis time can be decreased by a factor of 7 when compared to room temperature. From FTIR and 13C CP/MAS NMR analyses the increase in temperature causes a decrease in the number of carbonyl groups from carboxylic acid and from ionized carboxylic acid. The decarboxylation of d-Melanin monomers at higher temperatures shows that the use of higher synthesis temperatures influences the elimination of carbonyls present in the precursor molecules, thus facilitating the polymerization of d-Melanin.