Writers: Lorena Athie Goulart and Lucia Helena Mascaro
Keywords: Multi-walled carbon nanotubes; Nickel oxide nanoparticles; Phenolics; Electrodeposition; Simultaneous determination
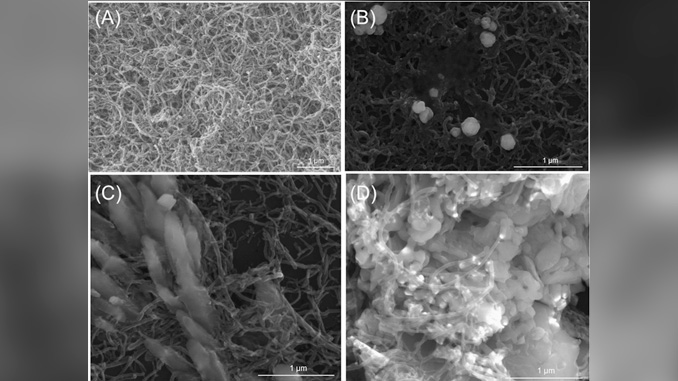
Abstract: This work reports the electrochemical determination of bisphenol A (BPA), hydroquinone (HQ) and catechol (CC) using glassy carbon electrode (GCE) modified with multi-walled carbon nanotubes (MWCNT) and nickel oxide nanoparticles (NiO). MWCNT were functionalized with sulfonitric solution (3H2SO4:1HNO3) and dispersed in dimethylformamide for the MWCNT/GCE manufacturing. The MWCNT/GCE was modified with NiO using cyclic potential in pH 4 maintained by an acetate buffer solution containing 0.008 mol L−1 of nickel nitrate. The concentration of the nickel solution and the number of cycles in the electrodeposition were studied. Morphological characterization of NiO/MWCNT/GCE was carried out by scanning electron microscopy and the presence of NiO was observed. The electrochemical behavior was evaluated by cyclic voltammetry and electrochemical impedance spectroscopy using BPA solution and the results were compared with those of GCE. The NiO/MWCNT/GCE presented the lowest charge transfer resistance. The electrochemical detection of BPA, HQ and CC was developed using differential pulse voltammetry. The analytical curves showed an excellent linear response and the detection limits for the simultaneous determination of BPA, HQ and CC were 2.8 × 10−8 mol L−1, 2.70 × 10−8 mol L−1 and 5.9 × 10−8 mol L−1, respectively.