Writers: Waldemir M. Carvalho Jr., Prof. Flavio L. Souza
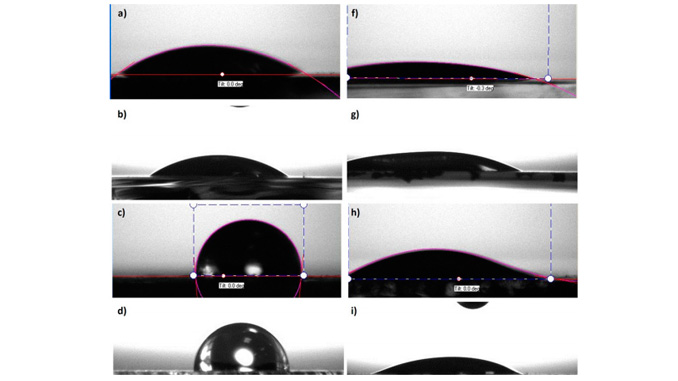
Abstract: In this study, the effect of tin (Sn4+) modification on the surface of hematite electrodes synthesized by an aqueous solution route at different times (2, 5, 10, 18, and 24 h) is investigated. As confirmed from X-ray diffraction results, the as-synthesized electrode exhibits an oxyhydroxide phase, which is converted into a pure hematite phase after being subjected to additional thermal treatment at 750 °C for 30 min. The tin-modified hematite electrode is prepared by depositing a solution of Sn4+ precursor on the as-synthesized electrode, followed by thermal treatment under the same abovementioned conditions. This modification results in an enhancement of the photocurrent response for all hematite electrodes investigated and attains the highest values of around 1.62 and 2.3 mA cm−2 at 1.23 and 1.4 V versus RHE, respectively, for electrodes obtained in short synthesis times (2 h). Contact angle measurements suggest that the deposition of Sn4+ on the hematite electrode provides a more hydrophilic surface, which favors a chemical reaction at the interface between the electrode and electrolyte. This result generates new perspectives for understanding the deposition of Sn4+ on the hematite electrode surface, which is in contrast with several studies previously reported; these studies state that the enhancement in photocurrent density is related to either the induction of an increased donor charge density or shift in the flat-band potential, which favors charge separation.