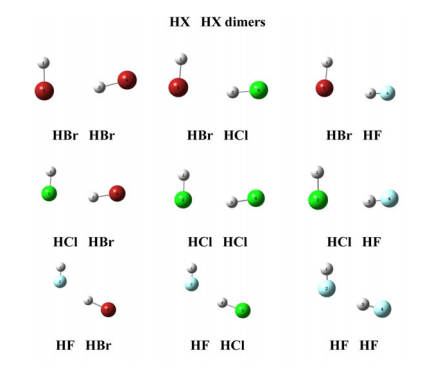
Quantum chemical topological analysis of hydrogen bonding in HX horizontal ellipsis HX and CH3X horizontal ellipsis HX dimers (X= Br, Cl, F)
Abstract: We present a systematic investigation of the nature and strength of the hydrogen bonding in HX center dot center dot center dot HX and CH3X horizontal ellipsis HX (X= Br, Cl and F) dimers using ab initio MP2/aug-cc-pVTZ calculations in the framework of the quantum theory of atoms in molecules (QTAIM) and electron localisation functions (ELFs) methods. The electron density of the complexes has been characterised, and the hydrogen bonding energy, as well as the QTAIM and ELF parameters, is consistent, providing deep insight into the origin of the hydrogen bonding in these complexes. It was found that in both linear and angular HX horizontal ellipsis HX and CH3X horizontal ellipsis HX dimers, F atoms form stronger HB than Br and Cl, but they need short (similar to 2 angstrom) X horizontal ellipsis HX contacts.
Author(s): Cormanich, RA; Santiago, RT; La Porta, FA; Freitas, MP; Rittner, R; da Cunha, EFF; Andres, J; Longo, E; Ramalho, TC
MOLECULAR SIMULATION
Volume: 41 Pages: 600-609 Published: MAY 3 2015
PDF: Quantum chemical topological analysis of hydrogen bonding in HX HX and CH3X HX dimers X Br Cl F
DOI: 10.1080/08927022.2014.904514