PtSn Electrocatalyst Supported on MWCNT-COOH: Investigating the Ethanol Oxidation Reaction
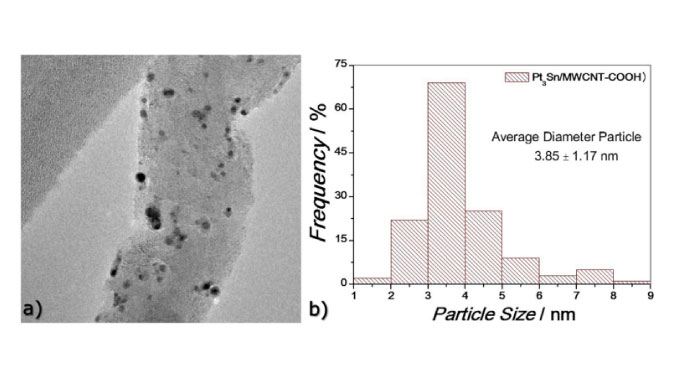
Abstract: Pt3Sn2 (a : a) electrocatalysts with 20% metal loading on multi-walled carbon nanotube supports functionalized with carboxylic acid groups (MWCNT-COOH) were prepared for studies on the ethanol oxidation reaction (EOR). Preparing and anchoring of the metallic nanoparticles increased the hydrophilicity of MWCNT-COOH and decreased its surface roughness to a value close to that of the commercial electrocatalyst Pt3Sn1/C E-TEK. Pt3Sn2/MWCNT-COOH consisted of 32% Pt3Sn alloy with a lattice parameter of 0.3979 nm. The mean particle size of 3.85 +/- 1.17 nm was measured by high-resolution transmission electron microscopy (HRTEM). The onset oxidation potential obtained for the EOR (in the cyclic voltammetry experiments) using Pt3Sn2/MWCNT-COOH was the lowest (0.21 V vs. reversible hydrogen electrode (RHE)), with a normalized current peak of 250 mAmg(Pt)(-1). The highest normalized current in the chronoamperometric measurements for the EOR after 1800 seconds at 0.5 V (RHE) was 16 mAmg(Pt)(-1), whereas for Pt3Sn1/C E-TEK it was 10 mAmg(Pt)(-1). FTIR-ATR in situ analysis showed that the Pt3Sn2/MWCNT-COOH electrocatalyst favoured acetaldehyde production at lower potentials and CO2 production at potentials greater than 0.5 V. In addition, the presence of oxygenated functional groups on the nanotube surfaces together with the anchoring of Pt and SnO2 formation contributed to the oxidation of ethanol to CO2 (bifunctional mechanism), enhancing the electrocatalytic activity of the material compared to commercial Pt3Sn1/C E-TEK.
Author(s): Parreira, Luanna Silveira; Martins Silva, Julio Cesar; Simoes, Fabio Ruiz; et al.
Chemelectrochem
Volume: 4 | Issue: 8 | Pages: 1950-1958 | Published: 2017
PDF: PtSn Electrocatalyst Supported on MWCNT-COOH: Investigating the Ethanol Oxidation Reaction
DOI: 10.1002/celc.201700326