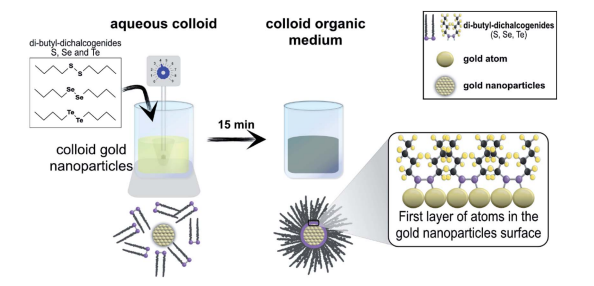
Stability of di-butyl-dichalcogenide-capped gold nanoparticles: experimental data and theoretical insights
Abstract: Metals capped with organochalcogenides have attracted considerable interest due to their practical applications, which include catalysis, sensing, and biosensing, due to their optical, magnetic, electrochemical, adhesive, lubrication, and antibacterial properties. There are numerous reports of metals capped with organothiol molecules; however, there are few studies on metals capped with organoselenium or organotellurium. Thus, there is a gap to be filled regarding the properties of organochalcogenide systems which can be improved by replacing sulfur with selenium or tellurium. In the last decade, there has been significant development in the synthesis of selenium and tellurium compounds; however, it is difficult to find commercial applications of these compounds because there are few studies showing the feasibility of their synthesis and their advantages compared to organothiol compounds. Stability against oxidation by molecular oxygen under ambient conditions is one of the properties which can be improved by choosing the correct organochalcogenide; this can confer important advantages for many more suitable applications. This paper reports the successful synthesis and characterization of gold nanoparticles functionalized with organochalcogenide molecules (dibutyl-disulfide, dibutyl-diselenide and dibutyl-ditelluride) and evaluates the oxidation stability of the organochalcogenides. Spherical gold nanoparticles with diameters of 24 nm were capped with organochalcogenides and were investigated using X-ray photoelectron spectroscopy (XPS) to show the improved stability of organoselenium compared with organothiol and organotellurium. The results suggest that the organoselenium is a promising candidate to replace organothiol because of its enhanced stability towards oxidation by molecular oxygen under ambient conditions and its slow oxidation rate. The observed difference in the oxidation processes, as discussed, is also in agreement with theoretical calculations.
Author(s): Gorup, L. F. ; Perlatti, B.; Kuznetsov, A.; Nascente, P.A.P.; Wendler, E. P.; Santos, A.A.; Barros, W.R.P.; Sequinel, T.; Tomitao, I.M.; Kubo, A.M.; Longo, E.; Camargo, E.R.
RSC Adv.
Published: 10 Feb 2020
DOI: https://doi.org/10.1039/C9RA07147D