Writers:
Keywords: Oxo–manganese complex; Electrochemical behavior; Enzymatic biomimicking; Electrocatalysis; Dopamine
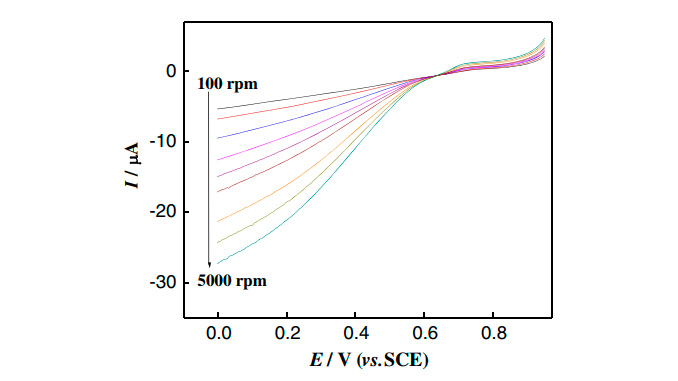
Abstract: This work describes the characterization of the [Mn2IV,IVO2(terpy)2(H2O)2]4+ complex in aqueous solution by UV–vis spectrophotometry, cyclic voltammetry, and linear sweep voltammetry with a rotating disk electrode. The pH effect, potential scan rate, effect of perfluorosulfonate polymer, and anion of supporting electrode on the electrochemical behavior of the modified electrode for better performance were investigated. The potential peak of the modified electrode was linearly dependent upon the ratio [ionic charge]/[ionic radius]. The modified electrode exerted an electrocatalytic effect on dopamine oxidation in aqueous solution with a decrease in the overpotential compared with the unmodified glassy carbon electrode. This way, the modified electrode showed an enzymatic biomimicking behavior. Tafel plot analyses were used to elucidate the kinetics and mechanism of dopamine oxidation.