α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) Solid Solutions: Structure, Morphology, and Optical Properties
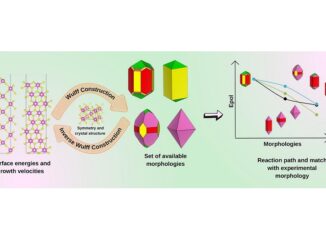
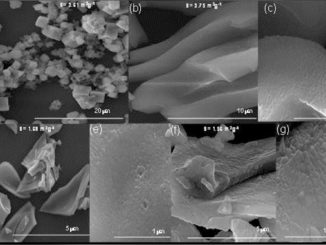
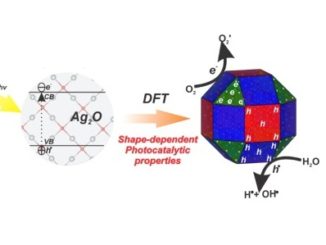
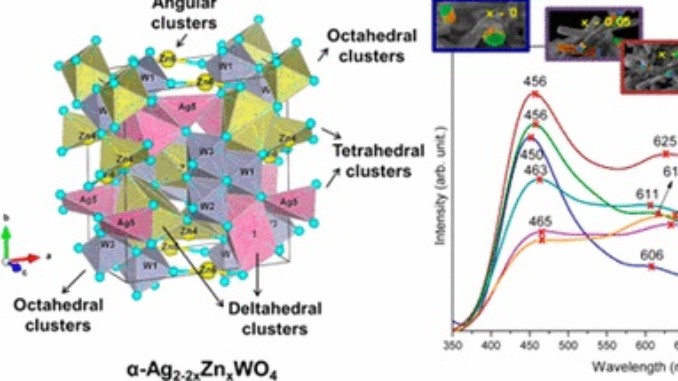
Abstract: A theoretical study was elaborated to support the experimental results of the Zn-doped α-Ag2WO4. Theses α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) solid solutions were obtained by coprecipitation method. X-ray diffraction data indicated that all α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) microcrystals presented an orthorhombic structure. The experimental values of the micro-Raman frequencies were in reasonable agreement with both previously reported and calculated results. Microscopy images showed that the replacement of Ag+ by Zn2+ promoted a reduction in the average crystal size and modifications in the morphology, from rod-like with hexagonal shape to roll-like with a curved surface. A theoretical methodology based on the surfaces calculations and Wulff constructions was applied to study the particle shapes transformations and the surface energy variations in α-Ag2–2xZnxWO4 (0 ≤ x ≤ 0.25) system. The decrease in the band gap value (from 3.18 to 3.08 eV) and the red shift in photoluminescence with the Zn2+ addition were associated with intermediary energy levels between the valence and conduction bands. First-principles calculations with density functional theory associated with B3LYP hybrid functional were conducted. The calculated band structures revealed an indirect band gap for the α-Ag2–2xZnxWO4 models. The electronic properties of α-Ag2WO4 and α-Ag2–2xZnxWO4 microcrystals were linked to distortion effects and oxygen vacancies (VOx) present in the clusters, respectively. Finally, photoluminescence properties of α-Ag2WO4 and α-Ag2–2xZnxWO4 microcrystals were explained by means of distortional effects and oxygen vacancies (VOx) in [AgOy] (y = 2, 4, 6, and 7) and [WO6] clusters, respectively, causing a red shift. Calculations revealed that the substitution for Ag+ with Zn2+occurred randomly in the α-Ag2WO4 lattice, and it was more favorable on the Ag4 site, where the local coordination of Ag+ cations was four.
Authors: Paula F. S. Pereira, Clayane C. Santos, Amanda F. Gouveia, Mateus M. Ferrer, Ivo M. Pinatti, Gleice Botelho, Julio R. Sambrano, Ieda L. V. Rosa, Juan Andrés & Elson Longo
Inorganic Chemistry
2017, 56 (13), pp 7360–7372
Link: https://pubs.acs.org/doi/ipdf/10.1021/acs.inorgchem.7b00201
DOI: 10.1021/acs.inorgchem.7b00201